An essential role for thymic mesenchyme in early T cell development
- PMID: 10727466
- PMCID: PMC2193125
- DOI: 10.1084/jem.191.6.1051
An essential role for thymic mesenchyme in early T cell development
Abstract
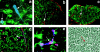
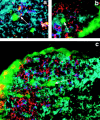
We show that the mesenchymal cells that surround the 12-d mouse embryo thymus are necessary for T cell differentiation. Thus, epithelial lobes with attached mesenchyme generate all T cell populations in vitro, whereas lobes from which mesenchyme has been removed show poor lymphopoiesis with few cells progressing beyond the CD4(-)CD8(-) stage of development. Interestingly, thymic mesenchyme is derived from neural crest cells, and extirpation of the region of the neural crest involved results in impaired thymic development and craniofacial abnormalities similar to the group of clinical defects found in the DiGeorge syndrome. Previous studies have suggested an inductive effect of mesenchyme on thymic epithelial morphogenesis. However, we have found that mesenchyme-derived fibroblasts are still required for early T cell development in the presence of mature epithelial cells, and hence mesenchyme might have a direct role in lymphopoiesis. We provide an anatomical basis for the role of mesenchyme by showing that mesenchymal cells migrate into the epithelial thymus to establish a network of fibroblasts and associated extracellular matrix. We propose that the latter might be important for T cell development through integrin and/or cytokine interactions with immature thymocytes.
Figures



References
-
- Cordier A.C., Haumont S.M. Development of thymus, parathyroids and ultimo-branchial bodies in NMRI and nude mice. Am. J. Anat. 1980;157:227–263. - PubMed
-
- Anderson G., Moore N.C., Owen J.J.T., Jenkinson E.J. Cellular interactions in thymocyte development. Annu. Rev. Immunol. 1996;14:73–99. - PubMed
-
- Le Lièvre C.S., Le Douarin N.M. Mesenchymal derivatives of the neural crestanalysis of chimeric quail and chick embryos. J. Embryol. Exp. Morphol. 1975;34:125–154. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

