Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells
- PMID: 10727467
- PMCID: PMC2193111
- DOI: 10.1084/jem.191.6.1057
Invariant chain controls H2-M proteolysis in mouse splenocytes and dendritic cells
Abstract
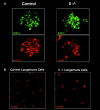
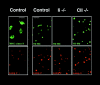
The association of invariant (Ii) chain with major histocompatibility complex (MHC) class II dimers is required for proper antigen presentation to T cells by antigen-presenting cells. Mice lacking Ii chain have severe abnormalities in class II transport, T cell selection, and B cell maturation. We demonstrate here that H2-M, which is required for efficient class II antigenic peptide loading, is unexpectedly downregulated in splenocytes and mature dendritic cells (DCs) from Ii(-/-) mice. Downregulation reflects an increased rate of degradation in Ii(-/-) cells. Degradation apparently occurs within lysosomes, as it is prevented by cysteine protease inhibitors such as E64, but not by the proteasome inhibitor lactacystin. Thus, Ii chain may act as a lysosomal protease inhibitor in B cells and DCs, with its deletion contributing indirectly to the loss of H2-M.
Figures



References
-
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 1994;12:259–293. - PubMed
-
- Wolf P.R., Ploegh H.L. How MHC class II molecules acquire peptide cargobiosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Biol. 1995;11:267–306. - PubMed
-
- Cresswell P. Invariant chain structure and MHC class II function. Cell. 1996;84:505–507. - PubMed
-
- Roche P.A. HLA-DMan in vivo facilitator of MHC class II peptide loading. Immunity. 1995;3:259–262. - PubMed
-
- Kropshofer H., Hammerling G.J., Vogt A.B. How HLA-DM edits the MHC class II peptide repertoiresurvival of the fittest? Immunol. Today. 1997;18:77–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

