Synaptic pathology in Borna disease virus persistent infection
- PMID: 10729116
- PMCID: PMC111850
- DOI: 10.1128/jvi.74.8.3441-3448.2000
Synaptic pathology in Borna disease virus persistent infection
Abstract
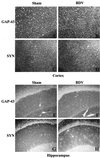
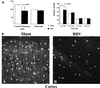
Borna disease virus (BDV) infection of newborn rats leads to a persistent infection of the brain, which is associated with behavioral and neuroanatonomical abnormalities. These disorders occur in the absence of lymphoid cell infiltrates, and BDV-induced cell damage is restricted to defined brain areas. To investigate if damage to synaptic structures anteceded neuronal loss in BDV neonatally infected rats, we analyzed at different times postinfection the expression levels of growth-associated protein 43 and synaptophysin, two molecules involved in neuroplasticity processes. We found that BDV induced a progressive and marked decrease in the expression of these synaptic markers, which was followed by a significant loss of cortical neurons. Our findings suggest that BDV persistent infection interferes with neuroplasticity processes in specific cell populations. This, in turn, could affect the proper supply of growth factors and other molecules required for survival of selective neuronal populations within the cortex and limbic system structures.
Figures
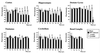
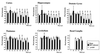

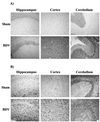
References
-
- Bautista J R, Rubin S A, Moran T H, Schwartz G J, Carbone K M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Dev Brain Res. 1995;90:45–53. - PubMed
-
- Bautista J R, Schwartz G J, de la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–36. - PubMed
-
- Bode L. Human infections with Borna disease virus and potential pathogenic implications. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 103–130. - PubMed
-
- Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. - PubMed
-
- Brahic M, Ozden S. Simultaneous detection of cellular RNA and proteins. In: Wilkinson R G, editor. In situ hybridization—a practical approach. Oxford, England: IRL Press; 1992. pp. 85–104.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

