Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB)
- PMID: 10729168
- PMCID: PMC111902
- DOI: 10.1128/jvi.74.8.3909-3917.2000
Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB)
Abstract
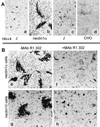
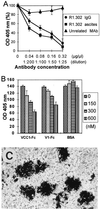
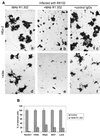
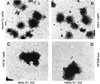
The immunoglobulin-like receptors that mediate entry of herpes simplex virus type 1 (HSV-1) into human cells were found to mediate the direct cell-to-cell spread of wild-type virus. The receptors here designated Nectin1alpha and -delta and Nectin2alpha were originally designated HIgR, PRR1/HveC, and PRR2alpha/HveB, respectively. We report the following. (i) Wild-type HSV-1 spreads from cell to cell in J cells expressing nectin1alpha or nectin1delta but not in parental J cells that are devoid of entry receptors. A monoclonal antibody to nectin1, which blocks entry, also blocked cell-to-cell spread in nectin1-expressing J cells. Moreover, wild-type virus did not spread from a receptor-positive to a receptor-negative cell. (ii) The antibody to nectin1 blocked transmission of wild-type virus in a number of human cell lines, with varying efficiencies, suggesting that nectin1 is the principal mediator of wild-type virus spread in a variety of human cell lines. (iii) Nectin1 did not mediate cell fusion induced by the syncytial strains HSV-1(MP) and HFEM-syn. (iv) Nectin2alpha could serve as a receptor for spread of a mutant virus carrying the L25P substitution in glycoprotein D, but not of wild-type virus, in agreement with its ability to mediate entry of the mutant but not of wild-type virus.
Figures

Similar articles
-
Nectin2alpha (PRR2alpha or HveB) and nectin2delta are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D.J Virol. 2000 Feb;74(3):1267-74. doi: 10.1128/jvi.74.3.1267-1274.2000. J Virol. 2000. PMID: 10627537 Free PMC article.
-
The herpes simplex virus JMP mutant enters receptor-negative J cells through a novel pathway independent of the known receptors nectin1, HveA, and nectin2.J Virol. 2004 May;78(9):4720-9. doi: 10.1128/jvi.78.9.4720-4729.2004. J Virol. 2004. PMID: 15078954 Free PMC article.
-
Chimeric nectin1-poliovirus receptor molecules identify a nectin1 region functional in herpes simplex virus entry.J Virol. 2001 Sep;75(17):7987-94. doi: 10.1128/jvi.75.17.7987-7994.2001. J Virol. 2001. PMID: 11483743 Free PMC article.
-
The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells.Rev Med Virol. 2000 Sep-Oct;10(5):305-19. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. Rev Med Virol. 2000. PMID: 11015742 Review.
-
The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells.J Virol. 1998 Dec;72(12):9992-10002. doi: 10.1128/JVI.72.12.9992-10002.1998. J Virol. 1998. PMID: 9811737 Free PMC article.
Cited by
-
Requirement of interaction of nectin-1alpha/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1.J Virol. 2001 May;75(10):4734-43. doi: 10.1128/JVI.75.10.4734-4743.2001. J Virol. 2001. PMID: 11312345 Free PMC article.
-
The Interaction Mechanism Between Herpes Simplex Virus 1 Glycoprotein D and Host Antiviral Protein Viperin.Front Immunol. 2019 Dec 11;10:2810. doi: 10.3389/fimmu.2019.02810. eCollection 2019. Front Immunol. 2019. PMID: 31921110 Free PMC article.
-
Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins.J Cell Biol. 2000 Sep 4;150(5):1161-76. doi: 10.1083/jcb.150.5.1161. J Cell Biol. 2000. PMID: 10974003 Free PMC article.
-
Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in guinea pigs.PLoS Pathog. 2018 May 23;14(5):e1007095. doi: 10.1371/journal.ppat.1007095. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29791513 Free PMC article.
-
Regulation of the metastasis suppressor Nm23-H1 by tumor viruses.Naunyn Schmiedebergs Arch Pharmacol. 2015 Feb;388(2):207-24. doi: 10.1007/s00210-014-1043-8. Epub 2014 Sep 10. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25199839 Free PMC article. Review.
References
-
- Balan P, Davis-Poynter N, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoprotein gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. - PubMed
-
- Card J P, Enquist L W. Neurovirulence of pseudorabies virus. Crit Rev Neurobiol. 1995;9:137–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

