Neurocan is upregulated in injured brain and in cytokine-treated astrocytes
- PMID: 10729323
- PMCID: PMC6772249
- DOI: 10.1523/JNEUROSCI.20-07-02427.2000
Neurocan is upregulated in injured brain and in cytokine-treated astrocytes
Abstract
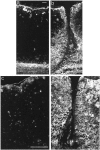
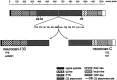
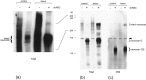
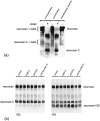
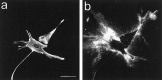
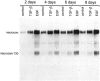
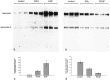
Injury to the CNS results in the formation of the glial scar, a primarily astrocytic structure that represents an obstacle to regrowing axons. Chondroitin sulfate proteoglycans (CSPG) are greatly upregulated in the glial scar, and a large body of evidence suggests that these molecules are inhibitory to axon regeneration. We show that the CSPG neurocan, which is expressed in the CNS, exerts a repulsive effect on growing cerebellar axons. Expression of neurocan was examined in the normal and damaged CNS. Frozen sections labeled with anti-neurocan monoclonal antibodies 7 d after a unilateral knife lesion to the cerebral cortex revealed an upregulation of neurocan around the lesion. Western blot analysis of extracts prepared from injured and uninjured tissue also revealed substantially more neurocan in the injured CNS. Western blot analysis revealed neurocan and the processed forms neurocan-C and neurocan-130 to be present in the conditioned medium of highly purified rat astrocytes. The amount detected was increased by transforming growth factor beta and to a greater extent by epidermal growth factor and was decreased by platelet-derived growth factor and, to a lesser extent, by interferon gamma. O-2A lineage cells were also capable of synthesizing and processing neurocan. Immunocytochemistry revealed neurocan to be deposited on the substrate around and under astrocytes but not on the cells. Astrocytes therefore lack the means to retain neurocan at the cell surface. These findings raise the possibility that neurocan interferes with axonal regeneration after CNS injury.
Figures




References
-
- Asher R, Bignami A. Localization of hyaluronate in primary glial cell cultures derived from newborn rat brain. Exp Cell Res. 1991;195:401–411. - PubMed
-
- Asher R, Perides G, Vanderhaeghen J-J, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res. 1991;28:410–421. - PubMed
-
- Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. - PubMed
-
- Asher RA, Fidler PS, Rogers JH, Fawcett JW. TGFbeta stimulates neurocan synthesis in cultured rat astrocytes. Soc Neurosci Abstr. 1998;24:56.
-
- Asher RA, Morgenstern DA, Adcock KH, Rogers JH, Fawcett JW. Versican is up-regulated in CNS injury and is a product of O-2A lineage cells. Soc Neurosci Abstr. 1999;25:750.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
