Release-independent short-term synaptic depression in cultured hippocampal neurons
- PMID: 10729328
- PMCID: PMC6772230
- DOI: 10.1523/JNEUROSCI.20-07-02480.2000
Release-independent short-term synaptic depression in cultured hippocampal neurons
Abstract
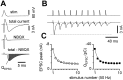
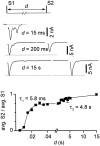
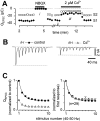
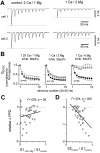
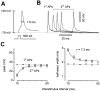
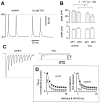
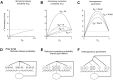
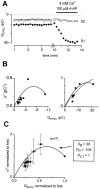
Short-term synaptic plasticity may dramatically influence neuronal information transfer, yet the underlying mechanisms remain incompletely understood. In autapses (self-synapses) formed by cultured hippocampal neurons, short-term synaptic depression (STD) had several unusual features. (1) Reduction of neurotransmitter release probability with Cd(2+), a blocker of voltage-gated calcium channels, did not change depression. (2) Lowering [Ca(2+)](o) and/or raising [Mg(2+)](o) had little effect on STD in cells with strong baseline depression, but in cells with more modest baseline depression, it reduced the depression. (3) Random variations in the size of initial EPSCs did not influence successive EPSC sizes. These findings were inconsistent with release-dependent mechanisms, such as vesicle depletion, post-synaptic receptor desensitization, and autoreceptor inhibition. Instead, other results suggested that changes in action potentials (APs) contributed to depression. The somatic APs declined in amplitude with repetitive stimulation, and modest reduction of AP amplitudes with tetrodotoxin inhibited EPSCs. Notably, tetrodotoxin also increased depression. Similar changes in axonal APs could produce STD in at least two ways. First, decreasing presynaptic spike amplitudes could reduce calcium entry and release probability. Alternatively, APs could fail to propagate through some axonal branches, reducing the number of active synapses. To explore these possibilities, we derived the expected variance of EPSCs for the two scenarios. Experimentally, the variance increased and then decreased on average with successive responses during trains of APs, confirming a unique prediction from the conduction failure scenario. Thus, STD had surprising properties, incompatible with commonly postulated mechanisms but consistent with AP conduction failure at axonal branches.
Figures

References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Alger BE, Teyler TJ. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976;110:463–480. - PubMed
-
- Banker GA, Cowan WM. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979;187:469–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
