The DNA binding protein BTEB mediates acetaldehyde-induced, jun N-terminal kinase-dependent alphaI(I) collagen gene expression in rat hepatic stellate cells
- PMID: 10733585
- PMCID: PMC85498
- DOI: 10.1128/MCB.20.8.2818-2826.2000
The DNA binding protein BTEB mediates acetaldehyde-induced, jun N-terminal kinase-dependent alphaI(I) collagen gene expression in rat hepatic stellate cells
Abstract
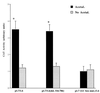
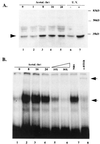
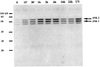
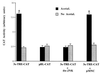
Alcohol-induced cirrhosis results partially from the excessive production of collagen matrix proteins, which, predominantly alphaI(I) collagen, are produced and secreted by activated hepatic stellate cells (HSC). The accumulation of alphaI(I) collagen in HSC during cirrhosis is largely due to an increase in alphaI(I) collagen gene expression. Acetaldehyde, the major active metabolite of alcohol, is known to stimulate alphaI(I) collagen production in HSC. However, the mechanisms responsible for it remain unknown. The aim of this study was to elucidate the mechanisms by which alphaI(I) collagen gene expression is induced by acetaldehyde in rat HSC. In the present study, the acetaldehyde response element was located in a distal GC box, previously described as the UV response element, in the promoter of the alphaI(I) collagen gene (-1484 to -1476). The GC box was predominantly bound by the DNA binding transcription factor BTEB (basic transcription element binding protein), expression of which was acetaldehyde and UV inducible. Blocking BTEB protein expression significantly reduced the steady-state levels of the acetaldehyde-induced alphaI(I) collagen mRNA, suggesting that BTEB is required for this gene expression. Further studies found that acetaldehyde activated Jun N-terminal kinase (JNK) 1 and 2 and activator protein 1 (AP-1) transactivating activity. Inhibition of JNK activation resulted in the reduction of the acetaldehyde-induced BTEB protein abundance and alphaI(I) collagen mRNA levels, indicating that the expression of both genes is JNK dependent in HSC. Taken together, these studies demonstrate that BTEB mediates acetaldehyde-induced, JNK-dependent alphaI(I) collagen gene expression in HSC.
Figures




References
-
- Bornstein P, McKay J. The first intron of the alpha 1(I) collagen gene contains several transcriptional regulatory elements. J Biol Chem. 1988;263:1603–1606. - PubMed
-
- Brenner D A, Chojkier M. Acetaldehyde increases collagen gene transcription in cultured human fibroblasts. J Biol Chem. 1987;262:17690–17695. - PubMed
-
- Casini A, Cunningham M, Rojkind M, Lieber C S. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758–765. - PubMed
-
- Casini A, Galli G, Salzano R, Ceni E, Franceschelli F, Rotella C M, Surrenti C. Acetaldehyde induces c-fos and c-jun proto-oncogenes in fat-storing cell cultures through protein kinase C activation. Alcohol Alcohol. 1994;29:303–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
