Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta
- PMID: 10733597
- PMCID: PMC85537
- DOI: 10.1128/MCB.20.8.2933-2940.2000
Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta
Abstract
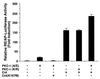
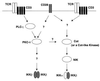
The NF-kappaB/Rel family of eukaryotic transcription factors plays an essential role in the regulation of inflammatory, antiapoptotic, and immune responses. NF-kappaB is activated by many stimuli including costimulation of T cells with ligands specific for the T-cell receptor (TCR)-CD3 complex and CD28 receptors. However, the signaling intermediates that transduce these costimulatory signals from the TCR-CD3 and CD28 surface receptors leading to nuclear NF-kappaB expression are not well defined. We now show that protein kinase C-theta (PKC-theta), a novel PKC isoform, plays a central role in a signaling pathway induced by CD3-CD28 costimulation leading to activation of NF-kappaB in Jurkat T cells. We find that expression of a constitutively active mutant of PKC-theta potently induces NF-kappaB activation and stimulates the RE/AP composite enhancer from the interleukin-2 gene. Conversely, expression of a kinase-deficient mutant or antisense PKC-theta selectively inhibits CD3-CD28 costimulation, but not tumor necrosis factor alpha-induced activation of NF-kappaB in Jurkat T cells. The induction of NF-kappaB by PKC-theta is mediated through the activation of IkappaB kinase beta (IKKbeta) in the absence of detectable IKKalpha stimulation. PKC-theta acts directly or indirectly to stimulate phosphorylation of IKKbeta, leading to activation of this enzyme. Together, these results implicate PKC-theta in one pathway of CD3-CD28 costimulation leading to NF-kappaB activation that is apparently distinct from that involving Cot and NF-kappaB-inducing kinase (NIK). PKC-theta activation of NF-kappaB is mediated through the selective induction of IKKbeta, while the Cot- and NIK-dependent pathway involves induction of both IKKalpha and IKKbeta.
Figures







References
-
- Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Baier G, Telford D, Giampa L, Coggeshall K M, Baier-Bitterlich G, Isakov N, Altman A. Molecular cloning and characterization of PKC-θ, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J Biol Chem. 1993;268:4997–5004. - PubMed
-
- Baldwin A J. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
