Molecular characterization of two-component systems of Helicobacter pylori
- PMID: 10735847
- PMCID: PMC111253
- DOI: 10.1128/JB.182.8.2068-2076.2000
Molecular characterization of two-component systems of Helicobacter pylori
Abstract
Two-component systems are frequently involved in the adaptation of bacteria to changing environmental conditions at the level of transcriptional regulation. Here we report the characterization of members of the two-component systems of the gastric pathogen Helicobacter pylori deduced from the genome sequence of strain 26695. We demonstrate that the response regulators HP166, HP1043, and HP1021 have essential functions, as disruption of the corresponding genes is lethal for the bacteria, irrespective of the fact that HP1043 and HP1021 have nonconserved substitutions in crucial amino acids of their receiver domains. An analysis of the in vitro phosphorylation properties of the two-component proteins demonstrates that HP244-HP703 and HP165-HP166 are cognate histidine kinase-response regulator pairs. Furthermore, we provide evidence that the variability of the histidine kinase HP165 caused by a poly(C) tract of variable length close to the 3' end of open reading frame 165/164 does not interfere with the kinase activity of the transmitter domain of HP165.
Figures
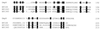

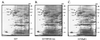


References
-
- Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. - PubMed
-
- Alm R A, Ling L-S L, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. - PubMed
-
- Appelmelk B J, Martin S L, Monteiro M A, Clayton C A, McColm A A, Zheng P, Verboom T, Maaskant J J, van den Eijnden D H, Hokke C H, Perry M B, Vandenbroucke-Grauls C M J E, Kusters J G. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in α3-fucosyltransferase genes. Infect Immun. 1999;67:5361–5366. - PMC - PubMed
-
- Appleby J L, Bourret R B. Activation of CheY mutant D57N by phosphorylation at an alternative site, Ser-56. Mol Microbiol. 1999;34:915–925. - PubMed
-
- Atherton J C, Cao P, Peek R M, Jr, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

