Permeation properties of inward-rectifier potassium channels and their molecular determinants
- PMID: 10736307
- PMCID: PMC2233762
- DOI: 10.1085/jgp.115.4.391
Permeation properties of inward-rectifier potassium channels and their molecular determinants
Abstract
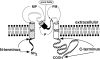
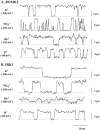
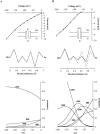
The structural domains contributing to ion permeation and selectivity in K channels were examined in inward-rectifier K(+) channels ROMK2 (Kir1.1b), IRK1 (Kir2.1), and their chimeras using heterologous expression in Xenopus oocytes. Patch-clamp recordings of single channels were obtained in the cell-attached mode with different permeant cations in the pipette. For inward K(+) conduction, replacing the extracellular loop of ROMK2 with that of IRK1 increased single-channel conductance by 25 pS (from 39 to 63 pS), whereas replacing the COOH terminus of ROMK2 with that of IRK1 decreased conductance by 16 pS (from 39 to 22 pS). These effects were additive and independent of the origin of the NH(2) terminus or transmembrane domains, suggesting that the two domains form two resistors in series. The larger conductance of the extracellular loop of IRK1 was attributable to a single amino acid difference (Thr versus Val) at the 3P position, three residues in front of the GYG motif. Permeability sequences for the conducted ions were similar for the two channels: Tl(+) > K(+) > Rb(+) > NH(4)(+). The ion selectivity sequence for ROMK2 based on conductance ratios was NH(4)(+) (1.6) > K(+) (1) > Tl(+) (0.5) > Rb(+) (0.4). For IRK1, the sequence was K(+) (1) > Tl(+) (0.8) > NH(4)(+) (0.6) >> Rb(+) (0.1). The difference in the NH(4)(+)/ K(+) conductance (1.6) and permeability (0.09) ratios can be explained if NH(4)(+) binds with lower affinity than K(+) to sites within the pore. The relatively low conductances of NH(4)(+) and Rb(+) through IRK1 were again attributable to the 3P position within the P region. Site-directed mutagenesis showed that the IRK1 selectivity pattern required either Thr or Ser at this position. In contrast, the COOH-terminal domain conferred the relatively high Tl(+) conductance in IRK1. We propose that the P-region and the COOH terminus contribute independently to the conductance and selectivity properties of the pore.
Figures




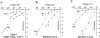



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

