Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca(2+) channel
- PMID: 10736313
- PMCID: PMC2233760
- DOI: 10.1085/jgp.115.4.467
Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca(2+) channel
Abstract
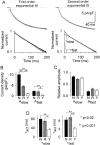
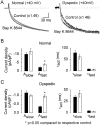
L-type Ca(2+) channel (L-channel) activity of the skeletal muscle dihydropyridine receptor is markedly enhanced by the skeletal muscle isoform of the ryanodine receptor (RyR1) (Nakai, J., R.T. Dirksen, H. T. Nguyen, I.N. Pessah, K.G. Beam, and P.D. Allen. 1996. Nature. 380:72-75.). However, the dependence of the biophysical and pharmacological properties of skeletal L-current on RyR1 has yet to be fully elucidated. Thus, we have evaluated the influence of RyR1 on the properties of macroscopic L-currents and intracellular charge movements in cultured skeletal myotubes derived from normal and "RyR1-knockout" (dyspedic) mice. Compared with normal myotubes, dyspedic myotubes exhibited a 40% reduction in the amount of maximal immobilization-resistant charge movement (Q(max), 7.5 +/- 0.8 and 4.5 +/- 0.4 nC/muF for normal and dyspedic myotubes, respectively) and an approximately fivefold reduction in the ratio of maximal L-channel conductance to charge movement (G(max)/Q(max)). Thus, RyR1 enhances both the expression level and Ca(2+) conducting activity of the skeletal L-channel. For both normal and dyspedic myotubes, the sum of two exponentials was required to fit L-current activation and resulted in extraction of the amplitudes (A(fast) and A(slow)) and time constants (tau(slow) and tau(fast)) for each component of the macroscopic current. In spite of a >10-fold in difference current density, L-currents in normal and dyspedic myotubes exhibited similar relative contributions of fast and slow components (at +40 mV; A(fast)/[A(fast) + A(slow)] approximately 0.25). However, both tau(fast) and tau(slow) were significantly (P < 0.02) faster for myotubes lacking the RyR1 protein (tau(fast), 8.5 +/- 1.2 and 4.4 +/- 0.5 ms; tau(slow), 79.5 +/- 10.5 and 34.6 +/- 3.7 ms at +40 mV for normal and dyspedic myotubes, respectively). In both normal and dyspedic myotubes, (-) Bay K 8644 (5 microM) caused a hyperpolarizing shift (approximately 10 mV) in the voltage dependence of channel activation and an 80% increase in peak L-current. However, the increase in peak L-current correlated with moderate increases in both A(slow) and A(fast) in normal myotubes, but a large increase in only A(fast) in dyspedic myotubes. Equimolar substitution of Ba(2+) for extracellular Ca(2+) increased both A(fast) and A(slow) in normal myotubes. The identical substitution in dyspedic myotubes failed to significantly alter the magnitude of either A(fast) or A(slow). These results demonstrate that RyR1 influences essential properties of skeletal L-channels (expression level, activation kinetics, modulation by dihydropyridine agonist, and divalent conductance) and supports the notion that RyR1 acts as an important allosteric modulator of the skeletal L-channel, analogous to that of a Ca(2+) channel accessory subunit.
Figures




References
-
- Adams B.A., Tanabe T., Mikami A., Numa S., Beam K.G. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature. 1990;346:569–572. - PubMed
-
- Armstrong C.M., Bezanilla F.M., Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetraacetic acid. Biochim. Biophys. Acta. 1972;267:605–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

