Dual regulation of calcium mobilization by inositol 1,4, 5-trisphosphate in a living cell
- PMID: 10736314
- PMCID: PMC2233754
- DOI: 10.1085/jgp.115.4.481
Dual regulation of calcium mobilization by inositol 1,4, 5-trisphosphate in a living cell
Abstract
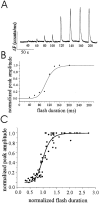
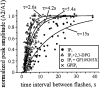
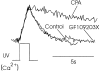
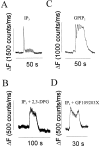
Changes in cytosolic free calcium ([Ca(2+)](i)) often take the form of a sustained response or repetitive oscillations. The frequency and amplitude of [Ca(2+)](i) oscillations are essential for the selective stimulation of gene expression and for enzyme activation. However, the mechanism that determines whether [Ca(2+)](i) oscillates at a particular frequency or becomes a sustained response is poorly understood. We find that [Ca(2+)](i) oscillations in rat megakaryocytes, as in other cells, results from a Ca(2+)-dependent inhibition of inositol 1,4,5-trisphosphate (IP(3))-induced Ca(2+) release. Moreover, we find that this inhibition becomes progressively less effective with higher IP(3) concentrations. We suggest that disinhibition, by increasing IP(3) concentration, of Ca(2+)-dependent inhibition is a common mechanism for the regulation of [Ca(2+)](i) oscillations in cells containing IP(3)-sensitive Ca(2+) stores.
Figures





Similar articles
-
Inhibition of inositol 1,4,5-trisphosphate-induced Ca2+ release by cAMP-dependent protein kinase in a living cell.Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1613-7. doi: 10.1073/pnas.95.4.1613. Proc Natl Acad Sci U S A. 1998. PMID: 9465064 Free PMC article.
-
Inhibitory effects of ruthenium red on inositol 1,4, 5-trisphosphate-induced responses in rat megakaryocytes.Biochem Pharmacol. 2001 Jan 1;61(1):7-13. doi: 10.1016/s0006-2952(00)00519-0. Biochem Pharmacol. 2001. PMID: 11137703
-
Ca2+ spike initiation from sensitized inositol 1,4,5-trisphosphate-sensitive Ca2+ stores in megakaryocytes.Pflugers Arch. 1994 Jun;427(3-4):355-64. doi: 10.1007/BF00374545. Pflugers Arch. 1994. PMID: 8072857
-
Inositol 1,4,5-trisphosphate and its receptors.Adv Exp Med Biol. 2012;740:255-79. doi: 10.1007/978-94-007-2888-2_11. Adv Exp Med Biol. 2012. PMID: 22453946 Review.
-
Coding and decoding of oscillatory Ca2+ signals.Semin Cell Dev Biol. 2019 Oct;94:11-19. doi: 10.1016/j.semcdb.2019.01.008. Epub 2019 Jan 25. Semin Cell Dev Biol. 2019. PMID: 30659886 Review.
Cited by
-
Altered Organelle Calcium Transport in Ovarian Physiology and Cancer.Cancers (Basel). 2020 Aug 10;12(8):2232. doi: 10.3390/cancers12082232. Cancers (Basel). 2020. PMID: 32785177 Free PMC article. Review.
References
-
- Auethavekiat V., Abrams C.S., Majerus P.W. Phosphorylation of platelet pleckstrin activates inositol polyphosphate 5-phosphatase I. J. Biol. Chem. 1997;272:1786–1790. - PubMed
-
- Berridge M.J., Bootman M.D., Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. - PubMed
-
- Berven L.A., Barritt G.J. A role for a pertussis toxin–sensitive trimeric G-protein in store-operated Ca2+ inflow in hepatocytes. FEBS Lett. 1994;346:235–240. - PubMed
-
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

