Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalis BFE 1071
- PMID: 10742203
- PMCID: PMC91984
- DOI: 10.1128/AEM.66.4.1298-1304.2000
Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B, two antimicrobial peptides produced by Enterococcus faecalis BFE 1071
Abstract
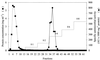
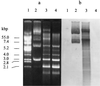
The pH-neutral cell supernatant of Enterococcus faecalis BFE 1071, isolated from the feces of minipigs in Göttingen, inhibited the growth of Enterococcus spp. and a few other gram-positive bacteria. Ammonium sulfate precipitation and cation-exchange chromatography of the cell supernatant, followed by mass spectrometry analysis, yielded two bacteriocin-like peptides of similar molecular mass: enterocin 1071A (4.285 kDa) and enterocin 1071B (3.899 kDa). Both peptides are always isolated together. The peptides are heat resistant (100 degrees C, 60 min; 50% of activity remained after 15 min at 121 degrees C), remain active after 30 min of incubation at pH 3 to 12, and are sensitive to treatment with proteolytic enzymes. Curing experiments indicated that the genes encoding enterocins 1071A and 1071B are located on a 50-kbp plasmid (pEF1071). Conjugation of plasmid pEF1071 to E. faecalis strains FA2-2 and OGX1 resulted in the expression of two active peptides with sizes identical to those of enterocins 1071A and 1071B. Sequencing of a DNA insert of 9 to 10 kbp revealed two open reading frames, ent1071A and ent1071B, which coded for 39- and 34-amino-acid peptides, respectively. The deduced amino acid sequence of the mature Ent1071A and Ent1071B peptides showed 64 and 61% homology with the alpha and beta peptides of lactococcin G, respectively. This is the first report of two new antimicrobial peptides representative of a fourth type of E. faecalis bacteriocin.
Figures




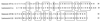

References
-
- Bhunia A K, Johnson M C, Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988;62:261–268. - PubMed
-
- Burger J H, Dicks L M T. Technique for isolating plasmids from exopolysaccharide producing Lactobacillus spp. Biotechnol Tech. 1994;8:769–772.
-
- Casaus P, Nilsen T, Cintas L M, Nes I F, Hernandez P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

