Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions
- PMID: 10742244
- PMCID: PMC92025
- DOI: 10.1128/AEM.66.4.1572-1579.2000
Compatible-solute-supported periplasmic expression of functional recombinant proteins under stress conditions
Abstract
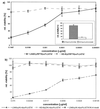
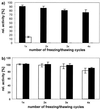
The standard method of producing recombinant proteins such as immunotoxins (rITs) in large quantities is to transform gram-negative bacteria and subsequently recover the desired protein from inclusion bodies by intensive de- and renaturing procedures. The major disadvantage of this technique is the low yield of active protein. Here we report the development of a novel strategy for the expression of functional rIT directed to the periplasmic space of Escherichia coli. rITs were recovered by freeze-thawing of pellets from shaking cultures of bacteria grown under osmotic stress (4% NaCl plus 0.5 M sorbitol) in the presence of compatible solutes. Compatible solutes, such as glycine betaine and hydroxyectoine, are low-molecular-weight osmolytes that occur naturally in halophilic bacteria and are known to protect proteins at high salt concentrations. Adding 10 mM glycine betaine for the cultivation of E. coli under osmotic stress not only allowed the bacteria to grow under these otherwise inhibitory conditions but also produced a periplasmic microenvironment for the generation of high concentrations of correctly folded rITs. Protein purified by combinations of metal ion affinity and size exclusion chromatography was substantially stabilized in the presence of 1 M hydroxyecotine after several rounds of freeze-thawing, even at very low protein concentrations. The binding properties and cytotoxic potency of the rITs were confirmed by competitive experiments. This novel compatible-solute-guided expression and purification strategy might also be applicable for high-yield periplasmic production of recombinant proteins in different expression systems.
Figures




References
-
- Altamiro M M, Garcia C, Possani L D, Fersht A R. Oxidative refolding chromatography: folding of the scorpion toxin Cn5. Nat Biotechnol. 1999;17:187–191. - PubMed
-
- Baneyx F, Georgiou G. Degradation of secreted proteins in Escherichia coli. Ann N Y Acad Sci. 1992;665:301–308. - PubMed
-
- Barth S, Huhn M, Wels W, Diehl V, Engert A. Construction and in vitro evaluation of RFT5(scFv)-ETA′, a new recombinant single-chain immunotoxin with specific cytotoxicity toward CD25+ Hodgkin-derived cell lines. Int J Mol Med. 1998;1:249–256. - PubMed
-
- Barth S, Matthey B, Huhn M, Diehl V, Engert A. CD30L-ETA′: a new recombinant immunotoxin based on the CD30 ligand for possible use against human lymphoma. Cytokines Cell Mol Ther. 1999;5:69–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

