gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes
- PMID: 10747014
- PMCID: PMC310215
- DOI: 10.1093/emboj/19.7.1458
gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes
Abstract
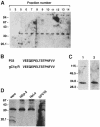
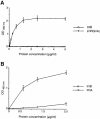
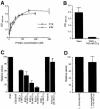
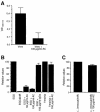
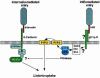
InlB is a Listeria monocytogenes protein that promotes entry of the bacterium into mammalian cells by stimulating tyrosine phosphorylation of the adaptor proteins Gab1, Cbl and Shc, and activation of phosphatidyl- inositol (PI) 3-kinase. Using affinity chromatography and enzyme-linked immunosorbent assay, we demonstrate a direct interaction between InlB and the mammalian protein gC1q-R, the receptor of the globular part of the complement component C1q. Soluble C1q or anti-gC1q-R antibodies impair InlB-mediated entry. Transient transfection of GPC16 cells, which are non-permissive to InlB-mediated entry, with a plasmid-expressing human gC1q-R promotes entry of InlB-coated beads. Furthermore, several experiments indicate that membrane recruitment and activation of PI 3-kinase involve an InlB-gC1q-R interaction and that gC1q-R associates with Gab1 upon stimulation of Vero cells with InlB. Thus, gC1q-R constitutes a cellular receptor involved in InlB-mediated activation of PI 3-kinase and tyrosine phosphorylation of the adaptor protein Gab1. After E-cadherin, the receptor for internalin, gC1q-R is the second identified mammalian receptor promoting entry of L. monocytogenes into mammalian cells.
Figures

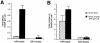

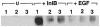
References
-
- Bobak D., Gaither, T., Frank, M. and Tenner, A.J. (1987) Modulation of FcR function by complement subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J. Immunol., 138, 1150–1156. - PubMed
-
- Braun L., Dramsi, S., Dehoux, P., Bierne, H., Lindahl, G. and Cossart, P. (1997) InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol. Microbiol., 25, 285–294. - PubMed
-
- Braun L., Ohayon, H. and Cossart, P. (1998) The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol., 27, 1077–1087. - PubMed
-
- Braun L., Nato, F., Payrastre, B., Mazié, J.-C. and Cossart, P. (1999) The 213-amino acid leucine-rich repeat region of the Listeria monocytogenes InlB protein is sufficient for entry into mammalian cells, stimulation of PI 3-kinase and membrane ruffling. Mol. Microbiol., 34, 10–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

