Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents
- PMID: 10747016
- PMCID: PMC310217
- DOI: 10.1093/emboj/19.7.1476
Highly purified glycosylphosphatidylinositols from Trypanosoma cruzi are potent proinflammatory agents
Abstract
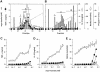
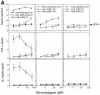
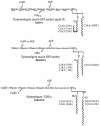
Intracellular protozoan parasites are potent stimulators of cell-mediated immunity. The induction of macrophage proinflammatory cytokines by Trypanosoma cruzi is considered to be important in controlling the infection and the outcome of Chagas' disease. Here we show that the potent tumour necrosis factor-alpha-, interleukin-12- and nitric oxide-inducing activities of T.cruzi trypomastigote mucins were recovered quantitatively in a highly purified and characterized glycosylphosphatidylinositol (GPI) anchor fraction of this material. The bioactive trypomastigote GPI fraction was compared with a relatively inactive GPI fraction prepared from T. cruzi epimastigote mucins. The trypomastigote GPI structures were found to contain additional galactose residues and unsaturated, instead of saturated, fatty acids in the sn-2 position of the alkylacylglycerolipid component. The latter feature is essential for the extreme potency of the trypomastigote GPI fraction, which is at least as active as bacterial endotoxin and Mycoplasma lipopeptide and, therefore, one of the most potent microbial proinflammatory agents known.
Figures



References
-
- Abbas A.K., Murphy, K.M. and Sher, A. (1996) Functional diversity of helper T lymphocytes. Nature, 383, 787–793. - PubMed
-
- Almeida I.C., Milani, S.R., Gorin, P.A. and Travassos, L.R. (1991) Complement-mediated lysis of Trypanosoma cruzi trypomastigotes by human anti-α-galactosyl antibodies. J. Immunol., 146, 2394–2400. - PubMed
-
- Almeida I.C., Ferguson, M.A.J., Schenkman, S. and Travassos, L.R. (1994) Lytic anti-α-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem. J., 304, 793–802. - PMC - PubMed
-
- Alves M.J.M. and Colli, W. (1975) Glycoproteins from Trypanosoma cruzi: partial purification by gel chromatography. FEBS Lett., 52, 188–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

