Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1
- PMID: 10747023
- PMCID: PMC310224
- DOI: 10.1093/emboj/19.7.1545
Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1
Abstract
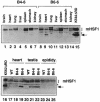
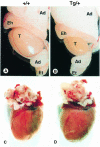
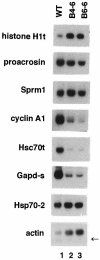
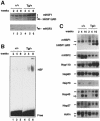
In mammals, testicular temperature is lower than core body temperature, and the vulnerable nature of spermatogenesis to thermal insult has been known for a century. However, the primary target affected by increases in temperature is not yet clear. We report here that male mice expressing an active form of heat shock transcription factor 1 (HSF1) in the testis are infertile due to a block in spermatogenesis. The germ cells entered meiotic prophase and were arrested at pachytene stage, and there was a significant increase in the number of apoptotic germ cells in these mice. In wild-type mice, a single heat exposure caused the activation of HSF1 and similar histological changes such as a stage-specific apoptosis of pachytene spermatocytes. These results suggest that male infertility caused by thermal insult is at least partly due to the activation of HSF1, which induces the primary spermatocytes to undergo apoptosis.
Figures






References
-
- Alastalo T.-P., Lönnström, M., Leppä, S., Kaarniranta, K., Pelto-Huikko, M., Sistonen, L. and Parvinen, M. (1998) Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp. Cell Res., 240, 16–27. - PubMed
-
- Bartell J.G., Davis, T., Kremer, E.J., Dewey, M.J. and Kistler, W.S. (1996) Expression of the rat testis-specific histone H1t gene in transgenic mice. J. Biol. Chem., 23, 4046–4054. - PubMed
-
- Chowdhury A.K. and Steinberger, E. (1970) Early changes in the germinal epithelium of rat testes following exposure to heat. J. Reprod. Fert., 22, 205–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

