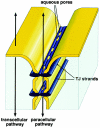
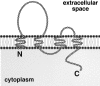
Pores in the wall: claudins constitute tight junction strands containing aqueous pores
- PMID: 10747082
- PMCID: PMC2175101
- DOI: 10.1083/jcb.149.1.13
Pores in the wall: claudins constitute tight junction strands containing aqueous pores
Figures
References
-
- Anderson J.M., van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. - PubMed
-
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049. - PMC - PubMed
-
- Briehl M.M., Miesfeld R.L. Isolation and characterization of transcripts induced by androgen withdrawal and apoptotic cell death in the rat ventral prostate. Mol. Endocrinol. 1991;5:1381–1388. - PubMed
-
- Bronstein J.M., Popper P., Micevych P.E., Farber D.B. Isolation and characterization of a novel oligodendrocyte-specific protein. Neurology. 1996;47:772–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

