Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis
- PMID: 10747083
- PMCID: PMC2175089
- DOI: 10.1083/jcb.149.1.17
Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis
Abstract
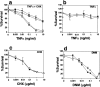
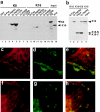
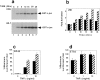
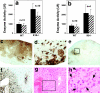
Tumor necrosis factor (TNF) is a cytokine produced by macrophages and T lymphocytes that acts through two distinct receptors, TNFR1 (60 kD, CD120a) and TNFR2 (80 kD, CD120b), to affect cellular proliferation, differentiation, survival, and cell death. In addition to its proinflammatory actions in mucosal tissue, TNF is important for liver regeneration. Keratin 8 (K8) and keratin 18 (K18) form intermediate filaments characteristic of liver and other single cell layered, internal epithelia and their derivative cancers. K8-deficient (K8(-)) mice, which escape embryonic lethality, develop inflammatory colorectal hyperplasia, mild liver abnormalities, and tolerate hepatectomy poorly. We show that normal and malignant epithelial cells deficient in K8 and K18 are approximately 100 times more sensitive to TNF-induced death. K8 and K18 both bind the cytoplasmic domain of TNFR2 and moderate TNF-induced, Jun NH(2)-terminal kinase (JNK) intracellular signaling and NFkappaB activation. Furthermore, K8(-) and K18(-) mice are much more sensitive to TNF dependent, apoptotic liver damage induced by the injection of concanavalin A. This moderation of the effects of TNF may be the fundamental function of K8 and K18 common to liver regeneration, inflammatory bowel disease, hepatotoxin sensitivity, and the diagnostic, persistent expression of these keratins in many carcinomas.
Figures

References
-
- Akerman P., Cote P., Yang S.Q., McClain C., Nelson S., Bagby G.J., Diehl A.M. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am. J. Physiol. 1992;263:G579–G585. - PubMed
-
- Ashkenazi A., Dixit V.M. Death receptorssignaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Baribault H., Price J., Miyai K., Oshima R.G. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993;7:1191–1202. - PubMed
-
- Baribault H., Penner J., Iozzo R.V., Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. - PubMed
-
- Blazka M.E., Wilmer J.L., Holladay S.D., Wilson R.E., Luster M.I. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 1995;133:43–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

