Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy
- PMID: 10747085
- PMCID: PMC2175107
- DOI: 10.1083/jcb.149.1.33
Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy
Abstract
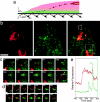
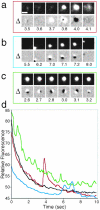
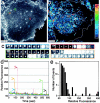
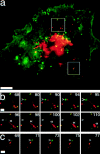
Monitoring the fusion of constitutive traffic with the plasma membrane has remained largely elusive. Ideally, fusion would be monitored with high spatial and temporal resolution. Recently, total internal reflection (TIR) microscopy was used to study regulated exocytosis of fluorescently labeled chromaffin granules. In this technique, only the bottom cellular surface is illuminated by an exponentially decaying evanescent wave of light. We have used a prism type TIR setup with a penetration depth of approximately 50 nm to monitor constitutive fusion of vesicular stomatitis virus glycoprotein tagged with the yellow fluorescent protein. Fusion of single transport containers (TCs) was clearly observed and gave a distinct analytical signature. TCs approached the membrane, appeared to dock, and later rapidly fuse, releasing a bright fluorescent cloud into the membrane. Observation and analysis provided insight about their dynamics, kinetics, and position before and during fusion. Combining TIR and wide-field microscopy allowed us to follow constitutive cargo from the Golgi complex to the cell surface. Our observations include the following: (1) local restrained movement of TCs near the membrane before fusion; (2) apparent anchoring near the cell surface; (3) heterogeneously sized TCs fused either completely; or (4) occasionally larger tubular-vesicular TCs partially fused at their tips.
Figures

References
-
- Axelrod D., Hellen E.H., Fulbright R.M. Total internal reflection fluorescence. In: Lakovicz J. R., editor. Topics in Fluorescence Spectrometry. Plenum Press; New York: 1992. p. 289.
-
- Betz W.J., Angleson J.K. Cellular secretion. Now you see it, now you don't. Nature. 1997;388:423–424. - PubMed
-
- Henry J.P., Darchen F., Cribier S. Physical techniques for the study of exocytosis in isolated cells. Biochimie. 1998;80:371–377. - PubMed

