Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition
- PMID: 10747095
- PMCID: PMC2175092
- DOI: 10.1083/jcb.149.1.167
Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition
Abstract
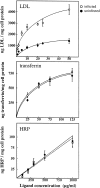
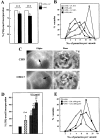
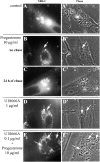
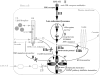
The obligate intracellular protozoan Toxoplasma gondii resides within a specialized parasitophorous vacuole (PV), isolated from host vesicular traffic. In this study, the origin of parasite cholesterol was investigated. T. gondii cannot synthesize sterols via the mevalonate pathway. Host cholesterol biosynthesis remains unchanged after infection and a blockade in host de novo sterol biosynthesis does not affect parasite growth. However, simultaneous limitation of exogenous and endogenous sources of cholesterol from the host cell strongly reduces parasite replication and parasite growth is stimulated by exogenously supplied cholesterol. Intracellular parasites acquire host cholesterol that is endocytosed by the low-density lipoprotein (LDL) pathway, a process that is specifically increased in infected cells. Interference with LDL endocytosis, with lysosomal degradation of LDL, or with cholesterol translocation from lysosomes blocks cholesterol delivery to the PV and significantly reduces parasite replication. Similarly, incubation of T. gondii in mutant cells defective in mobilization of cholesterol from lysosomes leads to a decrease of parasite cholesterol content and proliferation. This cholesterol trafficking to the PV is independent of the pathways involving the host Golgi or endoplasmic reticulum. Despite being segregated from the endocytic machinery of the host cell, the T. gondii vacuole actively accumulates LDL-derived cholesterol that has transited through host lysosomes.
Figures






References
-
- Bradfute D.L., Silva C.J., Simoni R.D. Squalene synthase-deficient mutant of Chinese hamster ovary cells. J. Biol. Chem. 1992;267:18308–18314. - PubMed
-
- Butler J.D., Blanchette-Mackie J., Goldin E., O'Neill R.R., Carstea G., Roff C.F., Patterson M.C., Patel S., Comly M.E., Cooney A., Vanier M.T., Brady R.O., Pentchev P.G. Progesterone blocks cholesterol translocation from lysosomes. J. Biol. Chem. 1992;267:23797–23805. - PubMed
-
- Coppens I., Andries M., Liu J.L., Cesbron-Delauw M.F. Intracellular trafficking of dense granule proteins in Toxoplasma gondii and experimental evidences for a regulated exocytosis. Eur. J. Cell Biol. 1999;78:463–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

