Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression
- PMID: 10748228
- PMCID: PMC2193179
- DOI: 10.1084/jem.191.7.1095
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression
Abstract
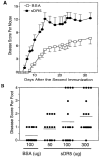
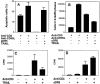
The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis of tumor cells but not normal cells; its role in normal nontransformed tissues is unknown. We report here that chronic blockade of TRAIL in mice exacerbated autoimmune arthritis, and that intraarticular TRAIL gene transfer ameliorated the disease. In vivo, TRAIL blockade led to profound hyperproliferation of synovial cells and arthritogenic lymphocytes and heightened the production of cytokines and autoantibodies. In vitro, TRAIL inhibited DNA synthesis and prevented cell cycle progression of lymphocytes. Interestingly, TRAIL had no effect on apoptosis of inflammatory cells either in vivo or in vitro. Thus, unlike other members of the tumor necrosis factor superfamily, TRAIL is a prototype inhibitor protein that inhibits autoimmune inflammation by blocking cell cycle progression.
Figures






References
-
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.P., Nicholl J.K., Sutherland G.R., Smith T.D., Rauch C., Smith C.A., Goodwin R.G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682 . - PubMed
-
- Pan G., Ni J., Wei Y.F., Yu G., Gentz R., Dixit V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818 . - PubMed
-
- Pan G., Ni J., Yu G., Wei Y.F., Dixit V.M. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41–45 . - PubMed
-
- Schneider P., Bodmer J.L., Thome M., Hofmann K., Holler N., Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997;416:329–334 . - PubMed
-
- Sheikh M.S., Burns T.F., Huang Y., Wu G.S., Amundson S., Brooks K.S., Fornace A.J., Jr., El-Deiry W.S. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

