Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans
- PMID: 10748241
- PMCID: PMC2193165
- DOI: 10.1084/jem.191.7.1241
Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans
Abstract
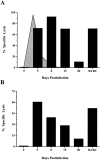
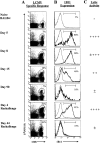
Currently there are few reliable cell surface markers that can clearly discriminate effector from memory T cells. To determine if there are changes in O-glycosylation between these two cell types, we analyzed virus-specific CD8 T cells at various time points after lymphocytic choriomeningitis virus infection of mice. Antigen-specific CD8 T cells were identified using major histocompatibility complex class I tetramers, and glycosylation changes were monitored with a monoclonal antibody (1B11) that recognizes O-glycans on mucin-type glycoproteins. We observed a striking upregulation of a specific cell surface O-glycan epitope on virus-specific CD8 T cells during the effector phase of the primary cytotoxic T lymphocyte (CTL) response. This upregulation showed a strong correlation with the acquisition of effector function and was downregulated on memory CD8 T cells. Upon reinfection, there was again increased expression of this specific O-glycan epitope on secondary CTL effectors, followed once more by decreased expression on memory cells. Thus, this study identifies a new cell surface marker to distinguish between effector and memory CD8 T cells. This marker can be used to isolate pure populations of effector CTLs and also to determine the proportion of memory CD8 T cells that are recruited into the secondary response upon reencounter with antigen. This latter information will be of value in optimizing immunization strategies for boosting CD8 T cell responses.
Figures


References
-
- Ahmed R., Gray D. Immunological memory and protective immunityunderstanding their relation. Science. 1996;272:54–60. - PubMed
-
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cellsa reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. - PubMed
-
- Buchmeier M.J., Zajac A.J. Lymphocytic choriomeningitis virus. In: Ahmed R., Chen I.S.Y., editors. Persistent Viral Infections. John Wiley & Sons; Chichester, UK: 1999. pp. 575–605.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

