A novel signaling mechanism between gas and blood compartments of the lung
- PMID: 10749570
- PMCID: PMC377480
- DOI: 10.1172/JCI8604
A novel signaling mechanism between gas and blood compartments of the lung
Erratum in
-
A novel signaling mechanism between gas and blood compartments of the lung.J Clin Invest. 2000 Aug;106(4):607. doi: 10.1172/jci8604c1. J Clin Invest. 2000. PMID: 10953036 Free PMC article. No abstract available.
Abstract
Propagation of inflammatory signals from the airspace to the vascular space is pivotal in lung inflammation, but mechanisms of intercompartmental signaling are not understood. To define signaling mechanisms, we microinfused single alveoli of blood-perfused rat lung with TNF-alpha, and determined in situ cytosolic Ca(2+) concentration ([Ca(2+)](i)) by the fura-2 ratio method, cytosolic phospholipase A(2) (cPLA(2)) activation and P-selectin expression by indirect immunofluorescence. Alveolar TNF-alpha increased [Ca(2+)](i) and activated cPLA(2) in alveolar epithelial cells, and increased both endothelial [Ca(2+)](i) and P-selectin expression in adjoining perialveolar capillaries. All responses were blocked by pretreating alveoli with a mAb against TNF receptor 1 (TNFR1). Crosslinking alveolar TNFR1 also increased endothelial [Ca(2+)](i). However, the endothelial responses to alveolar TNF-alpha were blocked by alveolar preinjection of the intracellular Ca(2+) chelator BAPTA-AM, or the cPLA(2) blockers AACOCF(3) and MAFP. The gap-junction uncoupler heptanol had no effect. We conclude that TNF-alpha induces signaling between the alveolar and vascular compartments of the lung. The signaling is attributable to ligation of alveolar TNFR1 followed by receptor-mediated [Ca(2+)](i) increases and cPLA(2) activation in alveolar epithelium. These novel mechanisms may be relevant in the alveolar recruitment of leukocytes.
Figures





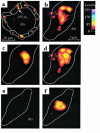


Comment in
-
Playing "telephone": bioactive lipids as mediators of intercompartmental communication in the alveolus.J Clin Invest. 2000 Apr;105(7):857-8. doi: 10.1172/JCI9724. J Clin Invest. 2000. PMID: 10749564 Free PMC article. No abstract available.
References
-
- Nicod LP. Pulmonary defense mechanisms. Respiration. 1999;66:2–11. - PubMed
-
- Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med. 1994;42:640–651. - PubMed
-
- Ward PA. Role of complement, chemokines, and regulatory cytokines in acute lung injury. Ann N Y Acad Sci. 1996;796:104–112. - PubMed
-
- Nelson S, et al. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammation response. J Infect Dis. 1989;159:189–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

