The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter
- PMID: 10749849
- PMCID: PMC2231805
- DOI: 10.1074/jbc.C000062200
The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter
Abstract
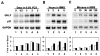
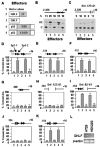
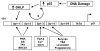
An important mechanism by which the tumor suppressor p53 maintains genomic stability is to induce cell cycle arrest through activation of the cyclin-dependent kinase inhibitor p21(WAF1/Cip1) gene. We show that the gene encoding the gut-enriched Krüppel-like factor (GKLF, KLF4) is concurrently induced with p21(WAF1/Cip1) during serum deprivation and DNA damage elicited by methyl methanesulfonate. The increases in expression of both Gklf and p21(WAF1/Cip1) due to DNA damage are dependent on p53. Moreover, during the first 30 min of methyl methanesulfonate treatment, the rise in Gklf mRNA level precedes that in p21(WAF1/Cip1), suggesting that GKLF may be involved in the induction of p21(WAF1/Cip1). Indeed, GKLF activates p21(WAF1/Cip1) through a specific Sp1-like cis-element in the p21(WAF1/Cip1) proximal promoter. The same element is also required by p53 to activate the p21(WAF1/Cip1) promoter, although p53 does not bind to it. Potential mechanisms by which p53 activates the p21(WAF1/Cip1) promoter include a physical interaction between p53 and GKLF and the transcriptional induction of Gklf by p53. Consequently, the two transactivators cause a synergistic induction of the p21(WAF1/Cip1) promoter activity. The physiological relevance of GKLF in mediating p53-dependent induction of p21(WAF1/Cip1) is demonstrated by the ability of antisense Gklf oligonucleotides to block the production of p21(WAF1/Cip1) in response to p53 activation. These findings suggest that GKLF is an essential mediator of p53 in the transcriptional induction of p21(WAF1/Cip1) and may be part of a novel pathway by which cellular responses to stress are modulated.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

