Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase
- PMID: 10749922
- PMCID: PMC14839
- DOI: 10.1091/mbc.11.4.1169
Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase
Abstract
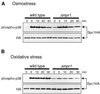
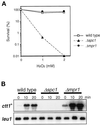
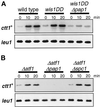
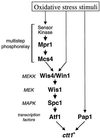
In response to oxidative stress, eukaryotic cells induce transcription of genes required for detoxification of oxidants. Here we present evidence that oxidative stress stimuli are transmitted by a multistep phosphorelay system to the Spc1/Sty1 stress-activated protein kinase in the fission yeast Schizosaccharomyces pombe. The fission yeast mpr1(+) gene encodes a novel protein with a histidine-containing phosphotransfer domain homologous to the budding yeast Ypd1. Spc1 activation upon oxidative stress is severely impaired in the Deltampr1 mutant as well as in the mpr1HQ strain, in which the putative phosphorylation site Mpr1-His221 is substituted with glutamine. In response to oxidative stress, Mpr1 binds to the Mcs4 response regulator that functions upstream of the Spc1 cascade, suggesting that Mcs4 is a cognate response regulator for Mpr1. Unexpectedly, when exposed to hydrogen peroxide, Deltampr1 cells can induce the catalase gene ctt1(+), one of the transcriptional targets of the Spc1 pathway, and survive oxidative stress in the absence of significant Spc1 activation. We have found that Pap1, a bZIP transcription factor homologous to human c-Jun, can mediate induction of ctt1(+) expression upon oxidative stress independently of the Spc1 stress-activated protein kinase. These studies show that oxidative stress stimuli are transmitted by multiple pathways to induce specific gene expression.
Figures






References
-
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1993.
-
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

