A dibasic motif in the tail of a class XIV apicomplexan myosin is an essential determinant of plasma membrane localization
- PMID: 10749937
- PMCID: PMC14854
- DOI: 10.1091/mbc.11.4.1385
A dibasic motif in the tail of a class XIV apicomplexan myosin is an essential determinant of plasma membrane localization
Abstract
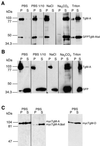
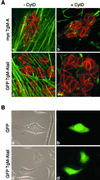
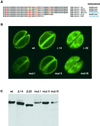
Obligate intracellular parasites of the phylum Apicomplexa exhibit gliding motility, a unique form of substrate-dependent locomotion essential for host cell invasion and shown to involve the parasite actin cytoskeleton and myosin motor(s). Toxoplasma gondii has been shown to express three class XIV myosins, TgM-A, -B, and -C. We identified an additional such myosin, TgM-D, and completed the sequences of a related Plasmodium falciparum myosin, PfM-A. Despite divergent structural features, TgM-A purified from parasites bound actin in an ATP-dependent manner. Isoform-specific antibodies revealed that TgM-A and recombinant mycTgM-A were localized right beneath the plasma membrane, and subcellular fractionation indicated a tight membrane association. Recombinant TgM-D also had a peripheral although not as sharply defined localization. Truncation of their respective tail domains abolished peripheral localization and tight membrane association. Conversely, fusion of the tails to green fluorescent protein (GFP) was sufficient to confer plasma membrane localization and sedimentability. The peripheral localization of TgM-A and of the GFP-tail fusion did not depend on an intact F-actin cytoskeleton, and the GFP chimera did not localize to the plasma membrane of HeLa cells. Finally, we showed that the specific localization determinants were in the very C terminus of the TgM-A tail, and site-directed mutagenesis revealed two essential arginine residues. We discuss the evidence for a proteinaceous plasma membrane receptor and the implications for the invasion process.
Figures






References
-
- Baker JP, Titus MA. A family of unconventional myosins from the nematode Caenorhabditis elegans. J Mol Biol. 1997;272:523–535. - PubMed
-
- Bement WM, Mooseker MS. TEDS rule: a molecular rationale for differential regulation of myosins by phosphorylation of the heavy chain head. Cell Motil Cytoskeleton. 1995;31:87–92. - PubMed
-
- Black M, Seeber F, Soldati D, Kim K, Boothroyd JC. Restriction enzyme-mediated integration elevates transformation frequency and enables co-transfection of Toxoplasma gondii. Mol Biochem Parasitol. 1995;74:55–63. - PubMed
-
- Brown SS. Myosins in yeast. Curr Opin Cell Biol. 1997;9:44–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

