Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B
- PMID: 10751335
- PMCID: PMC1876872
- DOI: 10.1016/s0002-9440(10)64980-2
Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B
Figures


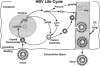





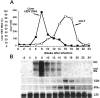

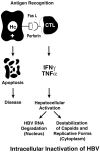
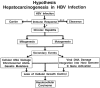
References
-
- Barker LF, Chisari FV, McGrath PP, Dalgard DW, Kirschstein RL, Almeida JD, Edington TS, Sharp DG, Peterson MR: Transmission of type B viral hepatitis to chimpanzees. J Infect Dis 1973, 127:648-662 - PubMed
-
- Chisari FV, Ferrari C: Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995, 13:29-60 - PubMed
-
- Beasley RP, Lin C-C, Hwang LY, Chen C-S: Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22,707 men in Taiwan. Lancet 1981, 2:1129-1133 - PubMed
-
- Chisari FV: Hepatitis B virus biology and pathogenesis. Friedmann T eds. Molecular Genetic Medicine. 1992, :pp 67-104 Academic Press, San Diego - PubMed
-
- Chisari FV, Ferrari C, Mondelli MU: Hepatitis B virus structure and biology. Microb Pathog 1989, 6:311-325 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

