Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia
- PMID: 10751355
- PMCID: PMC1876880
- DOI: 10.1016/S0002-9440(10)65000-6
Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia
Abstract
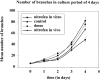
Pulmonary hypoplasia associated with congenital diaphragmatic hernia (CDH) remains a major therapeutic problem. Moreover, the pathogenesis of pulmonary hypoplasia in case of CDH is controversial. In particular, little is known about early lung development in this anomaly. To investigate lung development separate from diaphragm development we used an in vitro modification of the 2, 4-dichlorophenyl-p-nitrophenylether (Nitrofen) animal model for CDH. This enabled us to investigate the direct effects of Nitrofen on early lung development and branching morphogenesis in an organotypic explant system without the influence of impaired diaphragm development. Epithelial cell differentiation of the lung explants was assessed using surfactant protein-C and Clara cell secretory protein-10 mRNA expression as markers. Furthermore, cell proliferation and apoptosis were investigated. Our results indicate that Nitrofen negatively influences branching morphogenesis of the lung. Initial lung anlage formation is not affected. In addition, epithelial cell differentiation and cell proliferation are attenuated in lungs exposed to Nitrofen. These data indicate that Nitrofen interferes with early lung development before and separate from (aberrant) diaphragm development. Therefore, we postulate the dual-hit hypothesis, which explains pulmonary hypoplasia in CDH by two insults, one affecting both lungs before diaphragm development and one affecting the ipsilateral lung after defective diaphragm development.
Figures




References
-
- Torfs CP, Curry CJ, Bateson TF, Honore LH: A population-based study of congenital diaphragmatic hernia. Teratology 1992, 46:555-565 - PubMed
-
- Katz AL, Wiswell TE, Baumgart S: Contemporary controversies in the management of congenital diaphragmatic hernia. Clin Perinatol 1998, 25:219-248 - PubMed
-
- Reickert CA, Hirschl RB, Atkinson JB, Dudell G, Georgeson K, Glick P, Greenspan J, Kays D, Klein M, Lally KP, Mahaffey S, Ryckman F, Sawin R, Short BL, Stolar CJ, Thompson A, Wilson JM: Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery 1998, 123:305-310 - PubMed
-
- Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ: Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg 1995, 30:406-409 - PubMed
-
- Thebaud B, Mercier JC, Dinh-Xuan AT: Congenital diaphragmatic hernia: a cause of persistent pulmonary hypertension of the newborn which lacks an effective therapy. Biol Neonate 1998, 74:323-336 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

