Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin
- PMID: 10751360
- PMCID: PMC1876892
- DOI: 10.1016/s0002-9440(10)65005-5
Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin
Abstract
Angiogenesis depends on the cooperation of growth factors and cell adhesion events. Although alphav integrins have been shown to play critical roles in angiogenesis, recent studies in alphav-null mice suggest that other adhesion receptors and their ligands also regulate this process. Evidence is now provided that the integrin alpha5beta1 and its ligand fibronectin are coordinately up-regulated on blood vessels in human tumor biopsies and play critical roles in angiogenesis, resulting in tumor growth in vivo. Angiogenesis induced by multiple growth factors in chick embryos was blocked by monoclonal antibodies to the cell-binding domain of fibronectin. Furthermore, application of fibronectin or a proteolytic fragment of fibronectin containing the central cell-binding domain to the chick chorioallantoic membrane enhanced angiogenesis in an integrin alpha5beta1-dependent manner. Importantly, antibody, peptide, and novel nonpeptide antagonists of integrin alpha5beta1 blocked angiogenesis induced by several growth factors but had little effect on angiogenesis induced by vascular endothelial growth factor (VEGF) in both chick embryo and murine models. In fact, these alpha5beta1 antagonists inhibited tumor angiogenesis, thereby causing regression of human tumors in animal models. Thus, fibronectin and integrin alpha5beta1, like integrin alphavbeta3, contribute to an angiogenesis pathway that is distinct from VEGF-mediated angiogenesis, yet important for the growth of tumors.
Figures
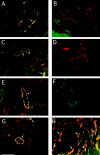
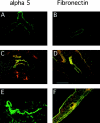
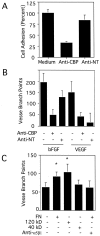
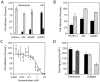
 ).
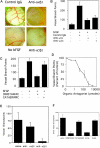
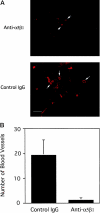
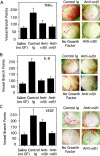
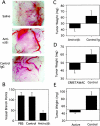
).

References
-
- Breier G, Risau W: The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol 1996, 6:454-456 - PubMed
-
- Breier G, Damert A, Plate KH, Risau W: Angiogenesis in embryos and ischemic diseases. Thromb Haemost 1997, 8:678-683 - PubMed
-
- Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 - PubMed
-
- Risau W: Mechanism of angiogenesis. Nature 1997, 386:671-674 - PubMed
-
- Stromblad S, Cheresh DA: Integrins, angiogenesis and vascular cell survival. Chem Biol 1996, 3:881-885 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

