Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo
- PMID: 10751370
- PMCID: PMC1876889
- DOI: 10.1016/S0002-9440(10)65015-8
Overexpression of VEGF 121 in immortalized endothelial cells causes conversion to slowly growing angiosarcoma and high level expression of the VEGF receptors VEGFR-1 and VEGFR-2 in vivo
Abstract
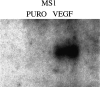
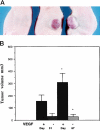
Vascular endothelial growth factor (VEGF or vascular permeability factor) is an important angiogenic factor that is up-regulated in numerous benign and malignant disorders, including angiosarcoma, hemangiomas, and solid tumors. To determine the functional role of VEGF in the development of endothelial tumors, we expressed primate VEGF 121 in an endothelial cell line, MS1, derived from primary murine cells by immortalization with a temperature-sensitive SV40 large T antigen. This cell line expresses the VEGFR-2 (Flk-1/Kdr) receptor for VEGF. Expression of VEGF 121 led to the development of slowly growing endothelial tumors, which were histologically well-differentiated angiosarcomas. The angiosarcomas generated from MS1 VEGF cells demonstrated up-regulation of the VEGF receptors VEGFR-2 and VEGFR-1 (Flt-1) in vivo compared with benign hemangiomas generated from MS1 cells. Treatment of these cells with the VEGFR-2 tyrosine kinase inhibitor SU 1498 led to decreased expression of ets-1, a transcription factor which has been shown to be stimulated by VEGF. These results suggest that high level expression of VEGF in endothelial cells may result in malignant transformation. This transformation process likely involves both autocrine and paracrine pathways.
Figures
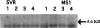




References
-
- Pratt GA: Birthmarks in infants. Arch Dermatol 1953, 67:302-305 - PubMed
-
- Vin-Christian K, McCalmont TH, Frieden IJ: Kaposiform hemangioendothelioma. A locally invasive vascular tumor that can mimic hemangioma of infancy. Arch Dermatol 1997, 133:1573-1578 - PubMed
-
- Morrison WH, Byers RM, Garden AS, Evans HL, Ang KK, Peters LJ: Cutaneous angiosarcoma of the head and neck: a therapeutic dilemma. Cancer 1995, 76:319-327 - PubMed
-
- Blei F, Karp N, Rofski N, Rosen R, Greco MA: Successful multimodal therapy for kaposiform hemangioendothelioma complicated by Kasabach-Merritt phenomenon: case report and review of the literature. Pediat Hematol Oncol 1998, 15:295-305 - PubMed
-
- Schmitz-Rixen T, Horsch S, Arnold G, Peters PE: Angiosarcoma in primary lymphedema of the lower extremity-Stewart-Treves syndrome. Lymphology 1984, 17:50-53 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

