Contrast gain control in the visual cortex: monocular versus binocular mechanisms
- PMID: 10751454
- PMCID: PMC6772209
- DOI: 10.1523/JNEUROSCI.20-08-03017.2000
Contrast gain control in the visual cortex: monocular versus binocular mechanisms
Abstract
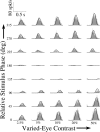
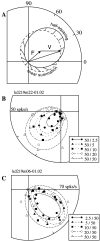
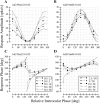
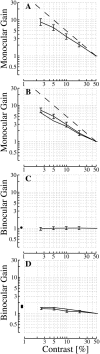
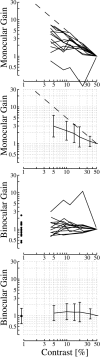
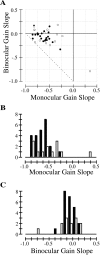
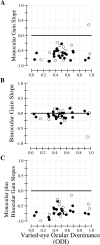
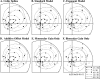
In this study, we compare binocular and monocular mechanisms underlying contrast encoding by binocular simple cells in primary visual cortex. At mid to high levels of stimulus contrast, contrast gain of cortical neurons typically decreases as stimulus contrast is increased (). We have devised a technique by which it is possible to determine the relative contributions of monocular and binocular processes to such reductions in contrast gain. First, we model the simple cell as an adjustable linear mechanism with a static output nonlinearity. For binocular cells, the linear mechanism is sensitive to inputs from both eyes. To constrain the parameters of the model, we record from binocular simple cells in striate cortex. To activate each cell, drifting sinusoidal gratings are presented dichoptically at various relative interocular phases. Stimulus contrast for one eye is varied over a large range whereas that for the other eye is fixed. We then determine the best-fitting parameters of the model for each cell for all of the interocular contrast ratios. This allows us to determine the effect of contrast on the contrast gain of the system. Finally, we decompose the contrast gain into monocular and binocular components. Using the data to constrain the model for a fixed contrast in one eye and increased contrasts in the other eye, we find steep reductions in monocular gain, whereas binocular gain exhibits modest and variable changes. These findings demonstrate that contrast gain reductions occur primarily at a monocular site, before convergence of information from the two eyes.
Figures



Similar articles
-
Contrast Normalization Accounts for Binocular Interactions in Human Striate and Extra-striate Visual Cortex.J Neurosci. 2020 Mar 25;40(13):2753-2763. doi: 10.1523/JNEUROSCI.2043-19.2020. Epub 2020 Feb 14. J Neurosci. 2020. PMID: 32060172 Free PMC article.
-
Contrast coding by cells in the cat's striate cortex: monocular vs. binocular detection.Vis Neurosci. 1995 Jan-Feb;12(1):77-93. doi: 10.1017/s0952523800007331. Vis Neurosci. 1995. PMID: 7718504
-
Binocular combination of contrast signals by striate cortical neurons in the monkey.J Neurophysiol. 1997 Jul;78(1):366-82. doi: 10.1152/jn.1997.78.1.366. J Neurophysiol. 1997. PMID: 9242286
-
Inter-ocular contrast normalization in human visual cortex.J Vis. 2009 Mar 20;9(3):13.1-22. doi: 10.1167/9.3.13. J Vis. 2009. PMID: 19757952 Free PMC article.
-
Binocular Integration in the Primate Primary Visual Cortex.Annu Rev Vis Sci. 2022 Sep 15;8:345-360. doi: 10.1146/annurev-vision-100720-112922. Epub 2022 Jun 8. Annu Rev Vis Sci. 2022. PMID: 35676095 Review.
Cited by
-
The Whole is Other Than the Sum: Perceived Contrast Summation Within Color and Luminance Plaids.Iperception. 2016 Oct 21;7(5):2041669516672481. doi: 10.1177/2041669516672481. eCollection 2016 Sep-Oct. Iperception. 2016. PMID: 27822354 Free PMC article.
-
Neural basis for stereopsis from second-order contrast cues.J Neurosci. 2006 Apr 19;26(16):4370-82. doi: 10.1523/JNEUROSCI.4379-05.2006. J Neurosci. 2006. PMID: 16624957 Free PMC article.
-
Mechanisms for stable, robust, and adaptive development of orientation maps in the primary visual cortex.J Neurosci. 2013 Oct 2;33(40):15747-66. doi: 10.1523/JNEUROSCI.1037-13.2013. J Neurosci. 2013. PMID: 24089483 Free PMC article.
-
Binocular summation of chance decisions.Sci Rep. 2015 Nov 18;5:16799. doi: 10.1038/srep16799. Sci Rep. 2015. PMID: 26577900 Free PMC article.
-
A unified model for binocular fusion and depth perception.Vision Res. 2021 Mar;180:11-36. doi: 10.1016/j.visres.2020.11.009. Epub 2020 Dec 21. Vision Res. 2021. PMID: 33359897 Free PMC article.
References
-
- Albrecht DG, Geisler WS. Motion sensitivity and the contrast-response function of simple cells in visual cortex. Vis Neurosci. 1991;7:531–546. - PubMed
-
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. - PubMed
-
- Anderson PA, Movshon JA. Binocular combination of contrast signals. Vision Res. 1989;29:1115–1132. - PubMed
-
- Anzai A, Bearse MA, Jr, Freeman RD, Cai D. Contrast coding by cells in the cat's striate cortex: monocular vs. binocular detection. Vis Neurosci. 1995;12:77–93. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
