Identical T cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph
- PMID: 10755885
- PMCID: PMC2195856
- DOI: 10.1084/jem.191.5.823
Identical T cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph
Abstract
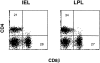
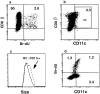
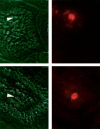
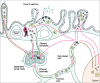
Murine gut intraepithelial (IEL) T cell receptor (TCR)-alpha/beta lymphocytes bearing CD8alpha/13 or CD8alpha/alpha coreceptors have been shown previously to express different oligoclonal TCR beta chain repertoires in the same mouse, in agreement with other evidence indicating that these two populations belong to different ontogenic lineages, with only CD8alpha/beta+ IELs being fully thymus dependent. CD8alpha/beta+, but not CD8alpha/alpha+, T lymphocytes are also present in the lamina propria. Here, we show that CD8alpha/beta+ lymphocytes from the lamina propria and the epithelium are both oligoclonal, and that they share the same TCR-beta clonotypes in the same mouse, as is also the case for CD4alpha T cells. Furthermore, identical T cell clones were detected among CD8alpha/beta IELs and CD8alpha/beta+ blasts circulating into the thoracic duct (TD) lymph of the same mouse, whereas TD small lymphocytes are polyclonal. These findings must be considered in light of previous observations showing that T blasts, but not small T lymphocytes, circulating in the TD lymph have the capacity of homing into the gut epithelium and lamina propria. These combined observations have interesting implications for our understanding of the recirculation of gut thymus-dependent lymphocytes and their precursors, and of the events leading up to the selection of their restricted TCR repertoire.
Figures






References
-
- Rocha B., Guy-Grand D., Vassalli P. Extrathymic T cell differentiation. Curr. Opin. Immunol. 1995;7:235–242. - PubMed
-
- Guy-Grand D., Cuenod-Jabri B., Malassis-Seris M., Selz F., Vassalli P. Complexity of the mouse gut T cell immune systemidentification of two distinct natural killer T cell intraepithelial lineages. Eur. J. Immunol. 1996;26:2246–2258. - PubMed
-
- Guy-Grand D., DiSanto J.P., Henchoz P., Malassis-Seris M., Vassalli P. Small bowel enteropathyrole of intraepithelial lymphocytes and of cytokines (IL12, IFNγ, TNF) in the induction of epithelial cell death and renewal. Eur. J. Immunol. 1998;28:730–744. - PubMed

