Zygomycetes in human disease
- PMID: 10756000
- PMCID: PMC100153
- DOI: 10.1128/CMR.13.2.236
Zygomycetes in human disease
Abstract
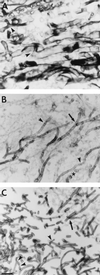
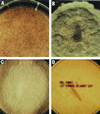
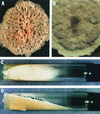
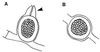
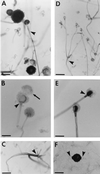
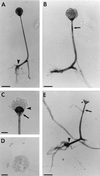
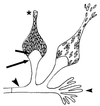
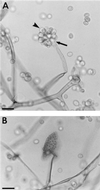
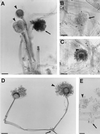
The Zygomycetes represent relatively uncommon isolates in the clinical laboratory, reflecting either environmental contaminants or, less commonly, a clinical disease called zygomycosis. There are two orders of Zygomycetes containing organisms that cause human disease, the Mucorales and the Entomophthorales. The majority of human illness is caused by the Mucorales. While disease is most commonly linked to Rhizopus spp., other organisms are also associated with human infection, including Mucor, Rhizomucor, Absidia, Apophysomyces, Saksenaea, Cunninghamella, Cokeromyces, and Syncephalastrum spp. Although Mortierella spp. do cause disease in animals, there is no longer sufficient evidence to suggest that they are true human pathogens. The spores from these molds are transmitted by inhalation, via a variety of percutaneous routes, or by ingestion of spores. Human zygomycosis caused by the Mucorales generally occurs in immunocompromised hosts as opportunistic infections. Host risk factors include diabetes mellitus, neutropenia, sustained immunosuppressive therapy, chronic prednisone use, iron chelation therapy, broad-spectrum antibiotic use, severe malnutrition, and primary breakdown in the integrity of the cutaneous barrier such as trauma, surgical wounds, needle sticks, or burns. Zygomycosis occurs only rarely in immunocompetent hosts. The disease manifestations reflect the mode of transmission, with rhinocerebral and pulmonary diseases being the most common manifestations. Cutaneous, gastrointestinal, and allergic diseases are also seen. The Mucorales are associated with angioinvasive disease, often leading to thrombosis, infarction of involved tissues, and tissue destruction mediated by a number of fungal proteases, lipases, and mycotoxins. If the diagnosis is not made early, dissemination often occurs. Therapy, if it is to be effective, must be started early and requires combinations of antifungal drugs, surgical intervention, and reversal of the underlying risk factors. The Entomophthorales are closely related to the Mucorales on the basis of sexual growth by production of zygospores and by the production of coenocytic hyphae. Despite these similarities, the Entomophthorales and Mucorales have dramatically different gross morphologies, asexual reproductive characteristics, and disease manifestations. In comparison to the floccose aerial mycelium of the Mucorales, the Entomophthorales produce a compact, glabrous mycelium. The asexually produced spores of the Entomophthorales may be passively released or actively expelled into the environment. Human disease with these organisms occurs predominantly in tropical regions, with transmission occurring by implantation of spores via minor trauma such as insect bites or by inhalation of spores into the sinuses. Conidiobolus typically infects mucocutaneous sites to produce sinusitis disease, while Basidiobolus infections occur as subcutaneous mycosis of the trunk and extremities. The Entomophthorales are true pathogens, infecting primarily immunocompetent hosts. They generally do not invade blood vessels and rarely disseminate. Occasional cases of disseminated and angioinvasive disease have recently been described, primarily in immunocompromised patients, suggesting a possible emerging role for this organism as an opportunist.
Figures


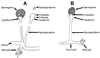
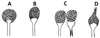




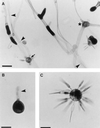

References
-
- Abdel-Hafez A I I, El-Sharouny H M M. Keratinophilic and saprophytic fungi isolated from student's nails in Egypt. J Basic Microbiol. 1990;30:3–11. - PubMed
-
- Abdel-Hafez A I I, El-Sharouny H M M. The occurrence of keratinophilic fungi in sewage sludge from Egypt. J Basic Microbiol. 1990;30:73–79. - PubMed
-
- Abdel-Hafez S I I. Composition of fungal flora of four cereal grains in Saudi Arabia. Mycopathologia. 1984;85:53–57. - PubMed
-
- Abdou R F, Magalla S E, Moharram A M, Abdel-Gawad K M, Sherif T H I, El-Syed Mahmood A L, Lottfy A E. Cytologic effects of fungal metabolites produced by fungi isolated from Egyptian poultry foodstuffs. J Basic Microbiol. 1989;29:131–139. - PubMed
-
- Abramowitz I. Fatal perforations of the stomach due to mucormycosis of the gastrointestinal tract. S Afr Med J. 1964;38:93–94. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

