Pathogenesis of intestinal amebiasis: from molecules to disease
- PMID: 10756002
- PMCID: PMC100155
- DOI: 10.1128/CMR.13.2.318
Pathogenesis of intestinal amebiasis: from molecules to disease
Abstract
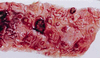
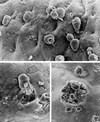
In spite of a wealth of knowledge on the biochemistry and cellular and molecular biology of Entamoeba histolytica, little has been done to apply these advances to our understanding of the lesions observed in patients with intestinal amebiasis. In this review, the pathological and histological findings in acute amebic colitis are related to the molecular mechanisms of E. histolytica pathogenicity described to date. Infection of the human colon by E. histolytica produces focal ulceration of the intestinal mucosa, resulting in dysentery (diarrhea with blood and mucus). Although a complete picture has not yet been achieved, the basic mechanisms involved in the production of focal lytic lesions include complex multifactorial processes in which lectins facilitate adhesion, proteases degrade extracellular matrix components, porins help nourish the parasite and may also kill incoming polymorphonuclear leukocytes and macrophages, and motility is used by the parasite to invade deeper layers of the colon. In addition, E. histolytica has developed mechanisms to modulate the immune response during acute infection. Nevertheless, much still needs to be unraveled to understand how this microscopic parasite has earned its well-deserved histolytic name.
Figures

References
-
- Adler P, Wood S J, Lee Y C, Lee R T, Petri W A, Jr, Schnaar R L. High affinity binding of the Entamoeba histolytica lectin to polyvalent N-acetylgalactosaminides. J Biol Chem. 1995;270:5164–5171. - PubMed
-
- Aguirre A, Warhurst D C, Guhl F, Frame I A. Polymerase chain reaction-solution hybridization enzyme-linked immunoassay (PCR-SHELA) for the differential diagnosis of pathogenic and non-pathogenic Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1995;89:187–188. - PubMed
-
- Aigner T, Neureiter D, Müller S, Küspert G, Belke J, Kirchner T. Extracellular matrix composition and gene expression in collagenous colitis. Gastroenterology. 1997;113:136–143. - PubMed
-
- Alon R N, Bracha R, Mirelman D. Transfection of Entamoeba dispar: inhibition of expression of the lysine-rich 30 kDa surface antigen by the transcription of its antisense RNA. Arch Med Res. 1997;28:S52–S55. - PubMed
-
- Andrä J, Leippe M. Pore-forming peptide of Entamoeba histolytica: significance of positively charged amino acid residues for its mode of action. FEBS Lett. 1994;354:97–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

