The vaccinia virus A14.5L gene encodes a hydrophobic 53-amino-acid virion membrane protein that enhances virulence in mice and is conserved among vertebrate poxviruses
- PMID: 10756020
- PMCID: PMC111922
- DOI: 10.1128/jvi.74.9.4085-4092.2000
The vaccinia virus A14.5L gene encodes a hydrophobic 53-amino-acid virion membrane protein that enhances virulence in mice and is conserved among vertebrate poxviruses
Abstract
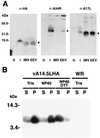
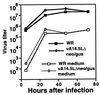
A short sequence, located between the A14L and A15L open reading frames (ORFs) of vaccinia virus, was predicted to encode a hydrophobic protein of 53 amino acids that is conserved in orthopoxviruses, leporipoxviruses, yatapoxiruses, and molluscipoxviruses. We constructed a recombinant vaccinia virus with a 10-codon epitope tag appended to the C terminus of the A14.5L ORF. Synthesis of the tagged protein occurred at late times and was blocked by an inhibitor of DNA replication, consistent with regulation by a predicted late promoter just upstream of the A14.5L ORF. Hydrophobicity of the protein was demonstrated by extraction into the detergent phase of Triton X-114. The protein was associated with purified vaccinia virus particles and with membranes of immature and mature virions that were visualized by electron microscopy of infected cells. Efficient release of the protein from purified virions occurred after treatment with a nonionic detergent and reducing agent. A mutant virus, in which the A14.5L ORF was largely deleted, produced normal-size plaques in several cell lines, and the yields of infectious intra- and extracellular viruses were similar to those of the parent. In contrast, with a mouse model, mutant viruses with the A14.5L ORF largely deleted were attenuated relative to that of the parental virus or a mutant virus with a restored A14.5L gene.
Figures






References
-
- Antoine G, Scheiflinger F, Dorner F, Falkner F G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–396. - PubMed
-
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1990;256:1604–1607. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

