Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A
- PMID: 10756022
- PMCID: PMC111924
- DOI: 10.1128/jvi.74.9.4102-4109.2000
Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A
Abstract
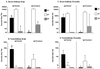
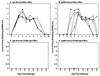
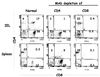
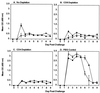
Immunoglobulin A (IgA) is the primary immune response induced in the intestine by rotavirus infection, but vaccination with virus-like particles induces predominantly IgG, not IgA. To definitively assess the role of IgA in protection from rotavirus infection, IgA knockout mice, which are devoid of serum and secretory IgA, were infected and then rechallenged with murine rotavirus at either 6 weeks or 10 months. Following primary rotavirus infection, IgA knockout mice cleared virus as effectively as IgA normal control mice. Rotavirus-infected IgA knockout mice produced no serum or fecal IgA but did have high levels of antirotavirus serum IgG and IgM and fecal IgG, whereas IgA normal control mice made both serum IgA and IgG and fecal IgA. Both IgA normal and IgA knockout mice were totally protected from rotavirus challenge at 42 days. Ten months following a primary infection, both IgA normal and knockout mice still had high levels of serum and fecal antirotavirus antibody and were totally protected from rotavirus challenge. To determine if compensatory mechanisms other than IgG were responsible for protection from rotavirus infection in IgA knockout mice, mice were depleted of CD4(+) T cells or CD8(+) T cells. No changes in the level of protection were seen in depleted mice. These data show that fecal or systemic IgA is not essential for protection from rotavirus infection and suggest that in the absence of IgA, IgG may play a significant role in protection from mucosal pathogens.
Figures
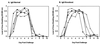

References
-
- Blanchard T G, Czinn S J, Redline R W, Sigmund N, Harriman G R, Nedrud J G. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 1999;191:74–80. - PubMed
-
- Coffin S E, Klinek M, Offit P A. Induction of virus-specific antibody production by lamina propria lymphocytes following intramuscular inoculation with rotavirus. J Infect Dis. 1995;172:874–878. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

