Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors
- PMID: 10756054
- PMCID: PMC111956
- DOI: 10.1128/jvi.74.9.4394-4403.2000
Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors
Abstract
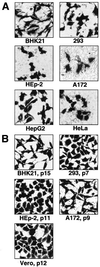
Primary features of the flavivirus Kunjin (KUN) subgenomic replicons include continuous noncytopathic replication in host cell cytoplasm and the ability to be encapsidated into secreted virus-like particles (VLPs). Previously we reported preparation of RNA-based KUN replicon vectors and expression of heterologous genes (HG) in cell culture after RNA transfection or after infection with recombinant KUN VLPs (A. N. Varnavski and A. A. Khromykh, Virology 255:366-375, 1999). In this study we describe the development of the next generation of KUN replicon vectors, which allow synthesis of replicon RNA in vivo from corresponding plasmid DNAs. These DNA-based vectors were able to direct stable expression of beta-galactosidase (beta-Gal) in several mammalian cell lines, and expression remained high ( approximately 150 pg per cell) throughout cell passaging. The applicability of these vectors in vivo was demonstrated by beta-Gal expression in the mouse lung epithelium for at least 8 weeks after intranasal inoculation and induction of anti-beta-Gal antibody response after intramuscular inoculation of the beta-Gal-encoding KUN replicon DNA. The noncytopathic nature of DNA-based KUN replicon vectors combined with high-level and stability of HG expression in a broad range of host cells should prove them to be useful in a variety of applications in vitro and in vivo.
Figures





Similar articles
-
Noncytopathic flavivirus replicon RNA-based system for expression and delivery of heterologous genes.Virology. 1999 Mar 15;255(2):366-75. doi: 10.1006/viro.1998.9564. Virology. 1999. PMID: 10069962
-
Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans.J Virol. 1998 Jul;72(7):5967-77. doi: 10.1128/JVI.72.7.5967-5977.1998. J Virol. 1998. PMID: 9621059 Free PMC article.
-
Tetracycline-inducible packaging cell line for production of flavivirus replicon particles.J Virol. 2004 Jan;78(1):531-8. doi: 10.1128/jvi.78.1.531-538.2004. J Virol. 2004. PMID: 14671135 Free PMC article.
-
Kunjin virus replicons: an RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications.Expert Opin Biol Ther. 2006 Feb;6(2):135-45. doi: 10.1517/14712598.6.2.135. Expert Opin Biol Ther. 2006. PMID: 16436039 Review.
-
Kunjin RNA replication and applications of Kunjin replicons.Adv Virus Res. 2003;59:99-140. doi: 10.1016/s0065-3527(03)59004-2. Adv Virus Res. 2003. PMID: 14696328 Review.
Cited by
-
Structure and function of the 3' terminal six nucleotides of the west nile virus genome in viral replication.J Virol. 2004 Aug;78(15):8159-71. doi: 10.1128/JVI.78.15.8159-8171.2004. J Virol. 2004. PMID: 15254187 Free PMC article.
-
Immunotherapy for cervical cancer: Research status and clinical potential.BioDrugs. 2010 Apr 1;24(2):109-29. doi: 10.2165/11532810-000000000-00000. BioDrugs. 2010. PMID: 20199126 Free PMC article. Review.
-
Therapeutic vaccines for high-risk HPV-associated diseases.Papillomavirus Res. 2018 Jun;5:46-58. doi: 10.1016/j.pvr.2017.12.006. Epub 2017 Dec 19. Papillomavirus Res. 2018. PMID: 29277575 Free PMC article. Review.
-
Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation.Virology. 2008 Nov 10;381(1):67-74. doi: 10.1016/j.virol.2008.08.021. Epub 2008 Sep 17. Virology. 2008. PMID: 18801552 Free PMC article.
-
Chimeric Zika viruses containing structural protein genes of insect-specific flaviviruses cannot replicate in vertebrate cells due to entry and post-translational restrictions.Virology. 2021 Jul;559:30-39. doi: 10.1016/j.virol.2021.03.014. Epub 2021 Mar 26. Virology. 2021. PMID: 33812340 Free PMC article.
References
-
- Altman-Hamamdzic S, Groseclose C, Ma J X, Hamamdzic D, Vrindavanam N S, Middaugh L D, Parratto N P, Sallee F R. Expression of beta-galactosidase in mouse brain: utilization of a novel nonreplicative Sindbis virus vector as a neuronal gene delivery system. Gene Ther. 1997;4:815–822. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Baker R T, Smith S A, Marano R, McKee J, Board P G. Protein expression using cotranslational fusion and cleavage of ubiquitin. Mutagenesis of the glutathione-binding site of human Pi class glutathione S-transferase. J Biol Chem. 1994;269:25381–25386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

