DNA strand transfer catalyzed by vaccinia topoisomerase: ligation of DNAs containing a 3' mononucleotide overhang
- PMID: 10756188
- PMCID: PMC103307
- DOI: 10.1093/nar/28.9.1893
DNA strand transfer catalyzed by vaccinia topoisomerase: ligation of DNAs containing a 3' mononucleotide overhang
Abstract
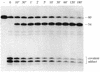
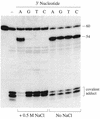
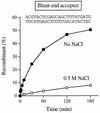
The specificity of vaccinia topoisomerase for transesterification to DNA at the sequence 5'-CCCTT and its versatility in strand transfer have illuminated the recombinogenic properties of type IB topoisomerases and spawned topoisomerase-based strategies for DNA cloning. Here we characterize a pathway of topoisomerase-mediated DNA ligation in which enzyme bound covalently to a CCCTT end with an unpaired +1T nucleotide rapidly and efficiently joins the CCCTT strand to a duplex DNA containing a 3' A overhang. The joining reaction occurs with high efficiency, albeit slowly, to duplex DNAs containing 3' G, T or C overhangs. Strand transfer can be restricted to the correctly paired 3' A overhang by including 0.5 M NaCl in the ligation reaction mixture. The effects of base mismatches and increased ionic strength on the rates of 3' overhang ligation provide a quantitative picture of the relative contributions of +1 T:A base pairing and electrostatic interactions downstream of the scissile phosphate to the productive binding of an unlinked acceptor DNA to the active site. The results clarify the biochemistry underlying topoisomerase-cloning of PCR products with non-templated 3' overhangs.
Figures



Similar articles
-
Recombinogenic flap ligation pathway for intrinsic repair of topoisomerase IB-induced double-strand breaks.Mol Cell Biol. 2000 Nov;20(21):8059-68. doi: 10.1128/MCB.20.21.8059-8068.2000. Mol Cell Biol. 2000. PMID: 11027276 Free PMC article.
-
Kinetic analysis of DNA and RNA strand transfer reactions catalyzed by vaccinia topoisomerase.J Biol Chem. 1997 Jun 20;272(25):15721-8. doi: 10.1074/jbc.272.25.15721. J Biol Chem. 1997. PMID: 9188465
-
Covalent DNA binding by vaccinia topoisomerase results in unpairing of the thymine base 5' of the scissile bond.J Biol Chem. 1996 Aug 9;271(32):19436-42. doi: 10.1074/jbc.271.32.19436. J Biol Chem. 1996. PMID: 8702632
-
Vaccinia virus DNA topoisomerase: a model eukaryotic type IB enzyme.Biochim Biophys Acta. 1998 Oct 1;1400(1-3):321-37. doi: 10.1016/s0167-4781(98)00144-4. Biochim Biophys Acta. 1998. PMID: 9748643 Review.
-
Mechanistic aspects of DNA topoisomerases.Adv Protein Chem. 1986;38:69-107. doi: 10.1016/s0065-3233(08)60526-4. Adv Protein Chem. 1986. PMID: 3026152 Review. No abstract available.
Cited by
-
A universal cloning vector using vaccinia topoisomerase I.Mol Biotechnol. 2006 May;33(1):23-8. doi: 10.1385/MB:33:1:23. Mol Biotechnol. 2006. PMID: 16691003
-
A highly efficient scheme for library preparation from single-stranded DNA.Sci Rep. 2023 Aug 25;13(1):13913. doi: 10.1038/s41598-023-40890-3. Sci Rep. 2023. PMID: 37626096 Free PMC article.
References
-
- Shuman S. (1998) Biochim. Biophys. Acta, 1400, 321–337. - PubMed
-
- Shuman S. and Prescott,J. (1990) J. Biol. Chem., 265, 17826–17836. - PubMed
-
- Shuman S. (1992) J. Biol. Chem., 267, 8620–8627. - PubMed
-
- Shuman S. (1992) J. Biol. Chem., 267, 16755–16758. - PubMed
-
- Sekiguchi J., Cheng,C. and Shuman,S. (1997) J. Biol. Chem., 272, 15721–15728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

