A novel cold-sensitive allele of the rate-limiting enzyme of fatty acid synthesis, acetyl coenzyme A carboxylase, affects the morphology of the yeast vacuole through acylation of Vac8p
- PMID: 10757783
- PMCID: PMC85561
- DOI: 10.1128/MCB.20.9.2984-2995.2000
A novel cold-sensitive allele of the rate-limiting enzyme of fatty acid synthesis, acetyl coenzyme A carboxylase, affects the morphology of the yeast vacuole through acylation of Vac8p
Abstract
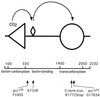
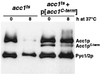
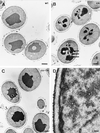
The yeast vacuole functions both as a degradative organelle and as a storage depot for small molecules and ions. Vacuoles are dynamic reticular structures that appear to alternately fuse and fragment as a function of growth stage and environment. Vac8p, an armadillo repeat-containing protein, has previously been shown to function both in vacuolar inheritance and in protein targeting from the cytoplasm to the vacuole. Both myristoylation and palmitoylation of Vac8p are required for its efficient localization to the vacuolar membrane (Y.-X. Wang, N. L. Catlett, and L. S. Weisman, J. Cell Biol. 140:1063-1074, 1998). We report that mutants with conditional defects in the rate-limiting enzyme of fatty acid synthesis, acetyl coenzyme A carboxylase (ACC1), display unusually multilobed vacuoles, similar to those observed in vac8 mutant cells. This vacuolar phenotype of acc1 mutant cells was shown biochemically to be accompanied by a reduced acylation of Vac8p which was alleviated by fatty acid supplementation. Consistent with the proposed defect of acc1 mutant cells in acylation of Vac8p, vacuolar membrane localization of Vac8p was impaired upon shifting acc1 mutant cells to nonpermissive condition. The function of Vac8p in protein targeting, on the other hand, was not affected under these conditions. These observations link fatty acid synthesis and availability to direct morphological alterations of an organellar membrane.
Figures




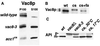
References
-
- Brown D A, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
