Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia
- PMID: 10758153
- PMCID: PMC18311
- DOI: 10.1073/pnas.070061297
Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia
Abstract
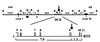
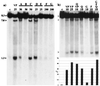
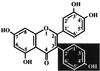
Chromosomal translocations involving the MLL gene occur in about 80% of infant leukemia. In the search for possible agents inducing infant leukemia, we identified bioflavonoids, natural substances in food as well as in dietary supplements, that cause site-specific DNA cleavage in the MLL breakpoint cluster region (BCR) in vivo. The MLL BCR DNA cleavage was shown in primary progenitor hematopoietic cells from healthy newborns and adults as well as in cell lines; it colocalized with the MLL BCR cleavage site induced by chemotherapeutic agents, such as etoposide (VP16) and doxorubicin (Dox). Both in vivo and additional in vitro experiments demonstrated topoisomerase II (topo II) as the target of bioflavonoids similar to VP16 and Dox. Based on 20 bioflavonoids tested, we identified a common structure essential for topo II-induced DNA cleavage. Reversibility experiments demonstrated a religation of the bioflavonoid as well as the VP16-induced MLL cleavage site. Our observations support a two-stage model of cellular processing of topo II inhibitors: The first and reversible stage of topo II-induced DNA cleavage results in DNA repair, but also rarely in chromosome translocations; whereas the second, nonreversible stage leads to cell death because of an accumulation of DNA damage. These results suggest that maternal ingestion of bioflavonoids may induce MLL breaks and potentially translocations in utero leading to infant and early childhood leukemia.
Figures
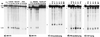

Comment in
-
Dietary flavonoids and the MLL gene: A pathway to infant leukemia?Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4411-3. doi: 10.1073/pnas.97.9.4411. Proc Natl Acad Sci U S A. 2000. PMID: 10781030 Free PMC article. Review. No abstract available.
Similar articles
-
An in vivo topoisomerase II cleavage site and a DNase I hypersensitive site colocalize near exon 9 in the MLL breakpoint cluster region.Blood. 1998 Nov 15;92(10):3793-803. Blood. 1998. PMID: 9808573
-
DNA structural properties of AF9 are similar to MLL and could act as recombination hot spots resulting in MLL/AF9 translocations and leukemogenesis.Hum Mol Genet. 2000 Jul 1;9(11):1671-9. doi: 10.1093/hmg/9.11.1671. Hum Mol Genet. 2000. PMID: 10861294
-
Polymorphisms in the MLL breakpoint cluster region (BCR).Hum Genet. 2003 Jul;113(1):80-91. doi: 10.1007/s00439-003-0936-2. Epub 2003 Mar 29. Hum Genet. 2003. PMID: 12665971
-
The MLL gene and translocations involving chromosomal band 11q23 in acute leukemia.Anticancer Res. 2005 May-Jun;25(3B):1931-44. Anticancer Res. 2005. PMID: 16158928 Review.
-
Topoisomerase II and the etiology of chromosomal translocations.DNA Repair (Amst). 2006 Sep 8;5(9-10):1093-108. doi: 10.1016/j.dnarep.2006.05.031. Epub 2006 Jul 20. DNA Repair (Amst). 2006. PMID: 16857431 Review.
Cited by
-
The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing.Nucleic Acids Res. 2009 Feb;37(3):738-48. doi: 10.1093/nar/gkn937. Epub 2008 Nov 28. Nucleic Acids Res. 2009. PMID: 19042970 Free PMC article. Review.
-
(-)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases.Chem Res Toxicol. 2008 Apr;21(4):936-43. doi: 10.1021/tx700434v. Epub 2008 Feb 23. Chem Res Toxicol. 2008. PMID: 18293940 Free PMC article.
-
Molecular mechanisms of topoisomerase 2 DNA-protein crosslink resolution.Cell Mol Life Sci. 2020 Jan;77(1):81-91. doi: 10.1007/s00018-019-03367-z. Epub 2019 Nov 15. Cell Mol Life Sci. 2020. PMID: 31728578 Free PMC article. Review.
-
Topoisomerase-mediated chromosomal break repair: an emerging player in many games.Nat Rev Cancer. 2015 Mar;15(3):137-51. doi: 10.1038/nrc3892. Epub 2015 Feb 19. Nat Rev Cancer. 2015. PMID: 25693836 Review.
-
Expression of MLL-AF4 or AF4-MLL fusions does not impact the efficiency of DNA damage repair.Oncotarget. 2016 May 24;7(21):30440-52. doi: 10.18632/oncotarget.8938. Oncotarget. 2016. PMID: 27119507 Free PMC article.
References
-
- Rowley J D. Annu Rev Genet. 1998;32:495–519. - PubMed
-
- Ross J A, Davies S M, Potter J D, Robison L L. Epidemiol Rev. 1994;16:243–272. - PubMed
-
- Canaani E, Nowell P C, Croce C M. Adv Cancer Res. 1995;66:213–234. - PubMed
-
- Liu L. Annu Rev Biochem. 1989;58:351–375. - PubMed
-
- Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier J M, Le Pecq J B, Larsen A K. Cancer Res. 1989;49:5111–5117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

