Local and distant protein structural changes on photoisomerization of the retinal in bacteriorhodopsin
- PMID: 10758159
- PMCID: PMC18286
- DOI: 10.1073/pnas.080064797
Local and distant protein structural changes on photoisomerization of the retinal in bacteriorhodopsin
Abstract
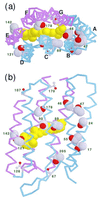
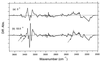
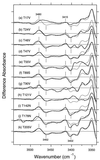
The photoisomerization of the retinal in bacteriorhodopsin is selective and efficient and yields perturbation of the protein structure within femtoseconds. The stored light energy in the primary intermediate is then used for the net translocation of a proton across the membrane in the microsecond to millisecond regime. This study is aimed at identifying how the protein changes on photoisomerization by using the O-H groups of threonines as internal probes. Polarized Fourier-transform IR spectroscopy of [3-(18)O]threonine-labeled and unlabeled bacteriorhodopsin indicates that 3 of the threonines (of a total of 18) change their hydrogen bonding. One is exchangeable in D(2)O, but two are not. A comprehensive mutation study indicates that the residues involved are Thr-89, Thr-17, and Thr-121 (or Thr-90). The perturbation of only three threonine side chains suggests that the structural alteration at this stage of the photocycle is local and specific. Furthermore, the structural change of Thr-17, which is located >11 A from the retinal chromophore, implicates a specific perturbation channel in the protein that accompanies the retinal motion.
Figures


Similar articles
-
Structural change of threonine 89 upon photoisomerization in bacteriorhodopsin as revealed by polarized FTIR spectroscopy.Biochemistry. 1999 Jul 27;38(30):9676-83. doi: 10.1021/bi990713y. Biochemistry. 1999. PMID: 10423246
-
Coupling of the reisomerization of the retinal, proton uptake, and reprotonation of Asp-96 in the N photointermediate of bacteriorhodopsin.Biochemistry. 2001 Sep 25;40(38):11308-17. doi: 10.1021/bi011027d. Biochemistry. 2001. PMID: 11560478
-
Partitioning of free energy gain between the photoisomerized retinal and the protein in bacteriorhodopsin.Biochemistry. 1998 Jul 14;37(28):9889-93. doi: 10.1021/bi980934o. Biochemistry. 1998. PMID: 9665693
-
Chemical dynamics in proteins: the photoisomerization of retinal in bacteriorhodopsin.Science. 1998 Mar 20;279(5358):1886-91. doi: 10.1126/science.279.5358.1886. Science. 1998. PMID: 9506931 Review.
-
Electrogenic processes and protein conformational changes accompanying the bacteriorhodopsin photocycle.Biochim Biophys Acta. 2000 Aug 30;1460(1):204-19. doi: 10.1016/s0005-2728(00)00140-7. Biochim Biophys Acta. 2000. PMID: 10984601 Review.
Cited by
-
FTIR study of primate color visual pigments.Biophysics (Nagoya-shi). 2015 Mar 4;11:61-6. doi: 10.2142/biophysics.11.61. eCollection 2015. Biophysics (Nagoya-shi). 2015. PMID: 27493516 Free PMC article. Review.
-
Protein-protein interaction changes in an archaeal light-signal transduction.J Biomed Biotechnol. 2010;2010:424760. doi: 10.1155/2010/424760. Epub 2010 Jun 29. J Biomed Biotechnol. 2010. PMID: 20671933 Free PMC article.
-
A sequence and structural study of transmembrane helices.J Comput Aided Mol Des. 2001 Jun;15(6):533-52. doi: 10.1023/a:1011197908960. J Comput Aided Mol Des. 2001. PMID: 11495225
-
Fourier-transform infrared study of the photoactivation process of Xenopus (6-4) photolyase.Biochemistry. 2012 Jul 24;51(29):5774-83. doi: 10.1021/bi300530x. Epub 2012 Jul 13. Biochemistry. 2012. PMID: 22747528 Free PMC article.
-
Comparison of the dynamics of the primary events of bacteriorhodopsin in its trimeric and monomeric states.Biophys J. 2002 Sep;83(3):1557-66. doi: 10.1016/S0006-3495(02)73925-8. Biophys J. 2002. PMID: 12202380 Free PMC article.
References
-
- Mathies R A, Lin S W, Ames J B, Pollard W T. Annu Rev Biophys Biophys Chem. 1991;20:491–518. - PubMed
-
- Ebrey T G. In: Thermodynamics of Membranes, Receptors and Channels. Jackson M, editor. New York: CRC; 1993. pp. 353–378.
-
- Lanyi J K. Biochim Biophys Acta. 1993;1183:241–261. - PubMed
-
- Haupts U, Tittor J, Oesterhelt D. Annu Rev Biophys Biomol Struct. 1999;28:367–399. - PubMed
-
- Grigorieff N, Ceska T A, Downing K H, Baldwin J M, Henderson R. J Mol Biol. 1996;259:393–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

