Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation
- PMID: 10758162
- PMCID: PMC18288
- DOI: 10.1073/pnas.080074897
Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation
Abstract
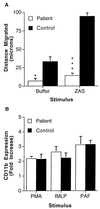
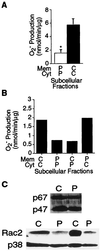
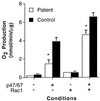
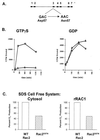
A 5-week-old male infant presented with severe bacterial infections and poor wound healing, suggesting a neutrophil defect. Neutrophils from this patient exhibited decreased chemotaxis, polarization, azurophilic granule secretion, and superoxide anion (O(2)(-)) production but had normal expression and up-regulation of CD11b. Rac2, which constitutes >96% of the Rac in neutrophils, is a member of the Rho family of GTPases that regulates the actin cytoskeleton and O(2)(-) production. Western blot analysis of lysates from patient neutrophils demonstrated decreased levels of Rac2 protein. Addition of recombinant Rac to extracts of the patient neutrophils reconstituted O(2)(-) production in an in vitro assay system. Molecular analysis identified a point mutation in one allele of the Rac2 gene resulting in the substitution of Asp57 by an Asn (Rac2(D57N)). Asp57 is invariant in all defined GTP-binding proteins. Rac2(D57N) binds GDP but not GTP and inhibits oxidase activation and O(2)(-) production in vitro. These data represent the description of an inhibitory mutation in a member of the Rho family of GTPases associated with a human immunodeficiency syndrome.
Figures
Similar articles
-
Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency.J Biol Chem. 2001 May 11;276(19):15929-38. doi: 10.1074/jbc.M010445200. Epub 2001 Feb 22. J Biol Chem. 2001. PMID: 11278678
-
Regulation of the human neutrophil NADPH oxidase by rho-related G-proteins.Biochemistry. 1993 Jun 1;32(21):5711-7. doi: 10.1021/bi00072a029. Biochemistry. 1993. PMID: 8504089
-
Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions.J Immunol. 2002 Nov 1;169(9):5043-51. doi: 10.4049/jimmunol.169.9.5043. J Immunol. 2002. PMID: 12391220
-
RAC2 GTPase deficiency and myeloid cell dysfunction in human and mouse.J Pediatr Hematol Oncol. 2002 Dec;24(9):791-4. doi: 10.1097/00043426-200212000-00027. J Pediatr Hematol Oncol. 2002. PMID: 12468931 Review.
-
Regulation of neutrophil function by Rac GTPases.Curr Opin Hematol. 2003 Jan;10(1):8-15. doi: 10.1097/00062752-200301000-00003. Curr Opin Hematol. 2003. PMID: 12483106 Review.
Cited by
-
Diagnosis of Chronic Granulomatous Disease: Strengths and Challenges in the Genomic Era.J Clin Med. 2024 Jul 29;13(15):4435. doi: 10.3390/jcm13154435. J Clin Med. 2024. PMID: 39124702 Free PMC article. Review.
-
Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis.Mol Cell Biol. 2005 May;25(10):4211-20. doi: 10.1128/MCB.25.10.4211-4220.2005. Mol Cell Biol. 2005. PMID: 15870290 Free PMC article.
-
Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase.Semin Immunopathol. 2008 Jul;30(3):255-71. doi: 10.1007/s00281-008-0119-2. Epub 2008 May 29. Semin Immunopathol. 2008. PMID: 18509648 Review.
-
Chronic Granulomatous Disease: a Comprehensive Review.Clin Rev Allergy Immunol. 2021 Oct;61(2):101-113. doi: 10.1007/s12016-020-08800-x. Clin Rev Allergy Immunol. 2021. PMID: 32524254 Review.
-
Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae.Eukaryot Cell. 2014 Oct;13(10):1266-77. doi: 10.1128/EC.00080-14. Epub 2014 May 30. Eukaryot Cell. 2014. PMID: 24879123 Free PMC article.
References
-
- Klebanoff S J, Clark R A. The Neutrophil: Functions and Clinical Disorders. Amsterdam: Elsevier; 1978.
-
- Curnutte J, Orkin S, Dinauer M. In: The Molecular Basis of Blood Diseases. 2nd Ed. Stamatoyannapoulos G, editor. Philadelphia: Saunders; 1994.
-
- Dharmawardhane S, Bokoch G M. Curr Opin Hematol. 1997;4:12–18. - PubMed
-
- Knause U G, Heyworth P G, Evans T, Curnutte J T, Bokoch G M. Science. 1991;254:1512–1515. - PubMed
-
- Leavey P J, Sellins K S, Thurman G, Elzi D, Hiester A, Silliman C C, Zerbe G, Cohen J J, Ambruso D R. Blood. 1998;92:4366–4374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

