Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity
- PMID: 10759498
- PMCID: PMC58937
- DOI: 10.1104/pp.122.4.1025
Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity
Abstract
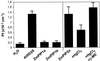
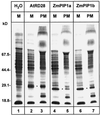
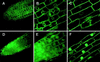
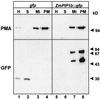
The transport of water through membranes is regulated in part by aquaporins or water channel proteins. These proteins are members of the larger family of major intrinsic proteins (MIPs). Plant aquaporins are categorized as either tonoplast intrinsic proteins (TIPs) or plasma membrane intrinsic proteins (PIPs). Sequence analysis shows that PIPs form several subclasses. We report on the characterization of three maize (Zea mays) PIPs belonging to the PIP1 and PIP2 subfamilies (ZmPIP1a, ZmPIP1b, and ZmPIP2a). The ZmPIP2a clone has normal aquaporin activity in Xenopus laevis oocytes. ZmPIP1a and ZmPIP1b have no activity, and a review of the literature shows that most PIP1 proteins identified in other plants have no or very low activity in oocytes. Arabidopsis PIP1 proteins are the only exception. Control experiments show that this lack of activity of maize PIP1 proteins is not caused by their failure to arrive at the plasma membrane of the oocytes. ZmPIP1b also does not appear to facilitate the transport of any of the small solutes tried (glycerol, choline, ethanol, urea, and amino acids). These results are discussed in relationship to the function and regulation of the PIP family of aquaporins.
Figures



References
-
- Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. - PubMed
-
- Barkla BJ, Vera-Estrella R, Pantoja O, Kirch HH, Bohnert HJ. Aquaporin localization: how valid are the TIP and PIP labels? Trends Plant Sci. 1999;4:86–88. - PubMed
-
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R. The Nicotiana tabacum membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J. 1999;18:565–570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

