Reduction and coordination of arsenic in Indian mustard
- PMID: 10759512
- PMCID: PMC58951
- DOI: 10.1104/pp.122.4.1171
Reduction and coordination of arsenic in Indian mustard
Abstract
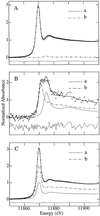
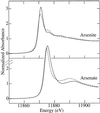
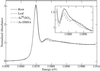
The bioaccumulation of arsenic by plants may provide a means of removing this element from contaminated soils and waters. However, to optimize this process it is important to understand the biological mechanisms involved. Using a combination of techniques, including x-ray absorption spectroscopy, we have established the biochemical fate of arsenic taken up by Indian mustard (Brassica juncea). After arsenate uptake by the roots, possibly via the phosphate transport mechanism, a small fraction is exported to the shoot via the xylem as the oxyanions arsenate and arsenite. Once in the shoot, the arsenic is stored as an As(III)-tris-thiolate complex. The majority of the arsenic remains in the roots as an As(III)-tris-thiolate complex, which is indistinguishable from that found in the shoots and from As(III)-tris-glutathione. The thiolate donors are thus probably either glutathione or phytochelatins. The addition of the dithiol arsenic chelator dimercaptosuccinate to the hydroponic culture medium caused a 5-fold-increased arsenic level in the leaves, although the total arsenic accumulation was only marginally increased. This suggests that the addition of dimercaptosuccinate to arsenic-contaminated soils may provide a way to promote arsenic bioaccumulation in plant shoots, a process that will be essential for the development of an efficient phytoremediation strategy for this element.
Figures



References
-
- Anderson CWN, Brooks RR, Stewart RB, Simcock R. Harvesting a crop of gold in plants. Nature. 1998;395:553–554.
-
- Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Tech. 1997;31:860–865.
-
- Carbonell-Barrachina A, Carbonell FB, Beneyto JM. Effect of arsenite on the concentrations of micronutrients in tomato plants grown in hydroponic culture. J Plant Nutr. 1994;17:1887–1903.
-
- Cox MS, Bell PF, Kovar JL. Differential tolerance of canola to arsenic when grown hydroponically or in soil. J Plant Nutr. 1996;19:1599–1610.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

