Programmed cell death during pollination-induced petal senescence in petunia
- PMID: 10759529
- PMCID: PMC58968
- DOI: 10.1104/pp.122.4.1323
Programmed cell death during pollination-induced petal senescence in petunia
Abstract
Petal senescence, one type of programmed cell death (PCD) in plants, is a genetically controlled sequence of events comprising its final developmental stage. We characterized the pollination-induced petal senescence process in Petunia inflata using a number of cell performance markers, including fresh/dry weight, protein amount, RNA amount, RNase activity, and cellular membrane leakage. Membrane disruption and DNA fragmentation with preferential oligonucleosomal cleavage, events characteristic of PCD, were found to be present in the advanced stage of petal senescence, indicating that plant and animal cell death phenomena share one of the molecular events in the execution phase. As in apoptosis in animals, both single-stranded DNase and double-stranded DNase activities are induced during petal cell death and are enhanced by Ca(2+). In contrast, the release of cytochrome c from mitochondria, one commitment step in signaling of apoptosis in animal cells, was found to be dispensable in petal cell death. Some components of the signal transduction pathway for PCD in plants are likely to differ from those in animal cells.
Figures



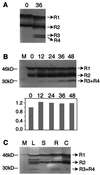



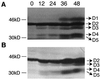
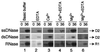
References
-
- Borochov A, Spiegelstein H, Philosoph HS. Ethylene and flower petal senescence: interrelationship with membrane lipid catabolism. Physiol Plant. 1997;100:606–612.
-
- Bortner CD, Oldenburg-Nicklas BE, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–26. - PubMed
-
- Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1998;1366:139–149. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

