Expression of allene oxide synthase determines defense gene activation in tomato
- PMID: 10759530
- PMCID: PMC58969
- DOI: 10.1104/pp.122.4.1335
Expression of allene oxide synthase determines defense gene activation in tomato
Abstract
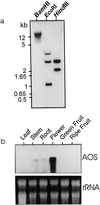
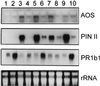
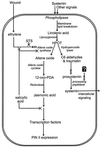
Allene oxide synthase (AOS; hydroperoxide dehydratase; EC 4.2.1.92) catalyzes the first step in the biosynthesis of jasmonic acid from lipoxygenase-derived hydroperoxides of free fatty acids. Using the AOS cDNA from tomato (Lycopersicon esculentum), in which the role of jasmonic acid in wound-induced defense gene activation has been best described, we examined the kinetics of AOS induction in response to wounding and elicitors, in parallel with that of the wound-inducible PIN II (proteinase inhibitor II) gene. AOS was induced in leaves by wounding, systemin, 12-oxophytodienoic acid, and methyl jasmonate. The levels of AOS mRNA started declining by 4 h after induction, whereas the levels of PIN II mRNA continued to increase up to 20 h after induction. Salicylic acid inhibited AOS and PIN II expression, and the addition of 12-oxophytodienoic acid or methyl jasmonate did not prevent the inhibition of PIN II expression in the presence of salicylic acid. Ethylene induced the expression of AOS, but the presence of ethylene alone did not produce an optimal induction of PIN II. The addition of silver thiosulfate, an ethylene action inhibitor, prevented the wound-induced expression of both AOS and PIN II. Products of hydroperoxide lyase affected neither AOS nor PIN II, but induced expression of prosystemin. Based on these results, we propose an updated model for defense gene activation in tomato.
Figures





References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

