Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species
- PMID: 10759531
- PMCID: PMC58970
- DOI: 10.1104/pp.122.4.1343
Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species
Abstract
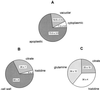
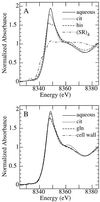
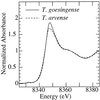
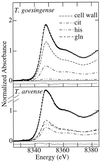
The ability of Thlaspi goesingense Hálácsy to hyperaccumulate Ni appears to be governed by its extraordinary degree of Ni tolerance. However, the physiological basis of this tolerance mechanism is unknown. We have investigated the role of vacuolar compartmentalization and chelation in this Ni tolerance. A direct comparison of Ni contents of vacuoles from leaves of T. goesingense and from the non-tolerant non-accumulator Thlaspi arvense L. showed that the hyperaccumulator accumulates approximately 2-fold more Ni in the vacuole than the non-accumulator under Ni exposure conditions that were non-toxic to both species. Using x-ray absorption spectroscopy we have been able to determine the likely identity of the compounds involved in chelating Ni within the leaf tissues of the hyperaccumulator and non-accumulator. This revealed that the majority of leaf Ni in the hyperaccumulator was associated with the cell wall, with the remaining Ni being associated with citrate and His, which we interpret as being localized primarily in the vacuolar and cytoplasm, respectively. This distribution of Ni was remarkably similar to that obtained by cell fractionation, supporting the hypothesis that in the hyperaccumulator, intracellular Ni is predominantly localized in the vacuole as a Ni-organic acid complex.
Figures
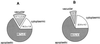
References
-
- Baker AJM, Brooks RR. Terrestrial higher plants which hyperaccumulate metallic elements: a review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126.
-
- Brune A, Urbach W, Dietz K-J. Compartmentation and transport of zinc in barley primary leaves as basic mechanisms involved in zinc tolerance. Plant Cell Environ. 1994;17:153–162.
-
- Brune A, Urbach W, Dietz K-J. Differential toxicity of heavy metals is partly related to a loss of preferential extraplasmatic compartmentation: a comparison of Cd-, Mo-, Ni-, and Zn-stress. New Phytol. 1995;129:403–409.
-
- Chaney RL. Plant uptake of inorganic waste. In: Parr JE, Marsh PB, Kla JM, editors. Land Treatment of Hazardous Wastes. Park Ridge, IL: Noyes Data; 1983. pp. 50–76.
-
- Dawson RMC, Elliott DC, Elliott WH, Jones KM, editors. Data for Biochemical Research. Ed 3. Oxford, UK: Clarendon Press; 1986.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

