Changes in Hechtian strands in cold-hardened cells measured by optical microsurgery
- PMID: 10759533
- PMCID: PMC58972
- DOI: 10.1104/pp.122.4.1365
Changes in Hechtian strands in cold-hardened cells measured by optical microsurgery
Abstract
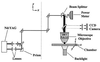
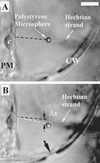
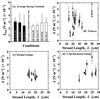
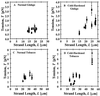
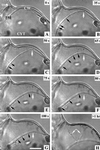
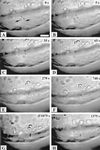
Optical microsurgical techniques were employed to investigate the mechanical properties of Hechtian strands in tobacco (Nicotiana tabacum) and Ginkgo biloba callus cells. Using optical tweezers, a 1. 5-microm diameter microsphere coated with concanavalin A was inserted though an ablated hole in the cell wall of a plasmolyzed cell and attached to a Hechtian strand. By displacing the adhered microsphere from equilibrium using the optical trapping force, the tensions of individual strands were determined. Measurements were made using both normal and cold-hardened cells, and in both cases, tensions were on the order of 10(-12) N. Significant differences were found in the binding strengths of cold-hardened and normal cultured cells. An increased number density of strands in cold-hardened G. biloba compared with normal cultured cells was also observed. Although no Hechtian strands were detected in any Arabidopsis callus cells, strands were present in leaf epidermal cells. Finally, the movement of attached microspheres was monitored along the outside of a strand while cycling the osmotic pressure.
Figures


References
-
- Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y. Efficient transformation of Arabidopsis thaliana: comparison of the efficiencies with various organs, plant ecotypes, and Agrobacterium strains. Plant Cell Rep. 1992;12:7–11. - PubMed
-
- Alberdi M, Corcuera LJ. Cold acclimation in plants. Phytochemistry. 1991;30:3177–3184.
-
- Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Observations of a single-beam gradient force optical trap for dielectric particles. Optics Lett. 1986;11:288–290. - PubMed
-
- Attree SM, Sheffield E. Plasmolysis of Pteridium protoplasts: a study using light and scanning-electron microscopy. Planta. 1985;165:151–157. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

