Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys
- PMID: 10759543
- PMCID: PMC18194
- DOI: 10.1073/pnas.050417697
Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys
Abstract
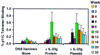
The potential utility of plasmid DNA as an HIV-1 vaccination modality currently is an area of active investigation. However, recent studies have raised doubts as to whether plasmid DNA alone will elicit immune responses of sufficient magnitude to protect against pathogenic AIDS virus challenges. We therefore investigated whether DNA vaccine-elicited immune responses in rhesus monkeys could be augmented by using either an IL-2/Ig fusion protein or a plasmid expressing IL-2/Ig. Sixteen monkeys, divided into four experimental groups, were immunized with (i) sham plasmid, (ii) HIV-1 Env 89.6P and simian immunodeficiency virus mac239 Gag DNA vaccines alone, (iii) these DNA vaccines and IL-2/Ig protein, or (iv) these DNA vaccines and IL-2/Ig plasmid. The administration of both IL-2/Ig protein and IL-2/Ig plasmid induced a significant and sustained in vivo activation of peripheral T cells in the vaccinated monkeys. The monkeys that received IL-2/Ig plasmid generated 30-fold higher Env-specific antibody titers and 5-fold higher Gag-specific, tetramer-positive CD8+ T cell levels than the monkeys receiving the DNA vaccines alone. IL-2/Ig protein also augmented the vaccine-elicited immune responses, but less effectively than IL-2/Ig plasmid. Augmentation of the immune responses by IL-2/Ig was evident after the primary immunization and increased with subsequent boost immunizations. These results demonstrate that the administration of IL-2/Ig plasmid can substantially augment vaccine-elicited humoral and cellular immune responses in higher primates.
Figures

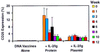



References
-
- United Nations Programme on HIV/AIDS (UNAIDS) Report on the global HIV/AIDS epidemic. 1998.
-
- Letvin N L. Science. 1998;280:1875–1880. - PubMed
-
- Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Science. 1990;247:1465–1468. - PubMed
-
- Tang D C, Devit M, Johnson S A. Nature (London) 1992;356:152–154. - PubMed
-
- Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Science. 1993;259:1745–1749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

